

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50166984 3-Methyl-2-(4-methyl-piperazin-1-yl)-quinoline-4-carboxylic acid [7-(1,2,3,4-tetrahydro-acridin-9-ylamino)-heptyl]-amide::3-methyl-2-(4-methylpiperazin-1-yl)-N-(7-(5,6,7,8-tetrahydroacridin-9-ylamino)heptyl)quinoline-4-carboxamide::CHEMBL195241
SMILES: CN1CCN(CC1)c1nc2ccccc2c(C(=O)NCCCCCCCNc2c3CCCCc3nc3ccccc23)c1C
InChI Key: InChIKey=ACKJXXOVSOCBPX-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50166984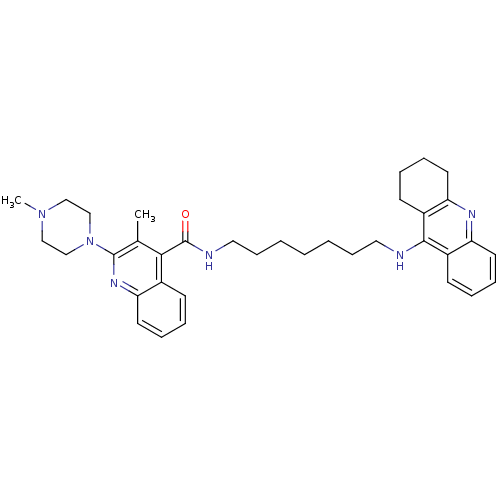 (3-Methyl-2-(4-methyl-piperazin-1-yl)-quinoline-4-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Binding affinity to 5HT3 receptor | J Med Chem 51: 347-72 (2008) Article DOI: 10.1021/jm7009364 BindingDB Entry DOI: 10.7270/Q25B039W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 3a (5-HT3a)/3b (5-HT3b) receptor (Rattus norvegicus-RAT) | BDBM50166984 (3-Methyl-2-(4-methyl-piperazin-1-yl)-quinoline-4-c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5-hydroxytryptamine 3 receptor of rat cortical membrane | J Med Chem 48: 3564-75 (2005) Article DOI: 10.1021/jm0493461 BindingDB Entry DOI: 10.7270/Q2HH6KTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin (5-HT) receptor (RAT) | BDBM50166984 (3-Methyl-2-(4-methyl-piperazin-1-yl)-quinoline-4-c...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5HT3 receptor in Wistar rat cortical membranes by liquid scintillation spectrometery | ACS Med Chem Lett 2: 571-576 (2011) Article DOI: 10.1021/ml2000388 BindingDB Entry DOI: 10.7270/Q2CZ385C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50166984 (3-Methyl-2-(4-methyl-piperazin-1-yl)-quinoline-4-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human AchE | J Med Chem 51: 347-72 (2008) Article DOI: 10.1021/jm7009364 BindingDB Entry DOI: 10.7270/Q25B039W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50166984 (3-Methyl-2-(4-methyl-piperazin-1-yl)-quinoline-4-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibitory concentration against human acetylcholinesterase | J Med Chem 48: 3564-75 (2005) Article DOI: 10.1021/jm0493461 BindingDB Entry DOI: 10.7270/Q2HH6KTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases (Homo sapiens (Human)) | BDBM50166984 (3-Methyl-2-(4-methyl-piperazin-1-yl)-quinoline-4-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibitory concentration against butyrylcholinesterase | J Med Chem 48: 3564-75 (2005) Article DOI: 10.1021/jm0493461 BindingDB Entry DOI: 10.7270/Q2HH6KTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases (Homo sapiens (Human)) | BDBM50166984 (3-Methyl-2-(4-methyl-piperazin-1-yl)-quinoline-4-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuchE | J Med Chem 51: 347-72 (2008) Article DOI: 10.1021/jm7009364 BindingDB Entry DOI: 10.7270/Q25B039W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||