

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50167935 CHEMBL195083::N-{4-[4-(3-chloro-2-methoxyphenyl)piperazin-1-yl]butyl}-1-ferra-1,1'-spirobi[pentacyclo[2.2.0.0^{1,3}.0^{1,5}.0^{2,6}]hexane]-2,2',4,4'-tetraene-6-carboxamide
SMILES: c1(Cl)c(c(N2CCN(CC2)CCCCNC(C23[Fe]456789%10%11C%12C4=C5C6=C7%12)=O)ccc1)OC.C8(C9=C2%10)=C3%11
InChI Key: InChIKey=RKIAQJFOXBVIPS-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50167935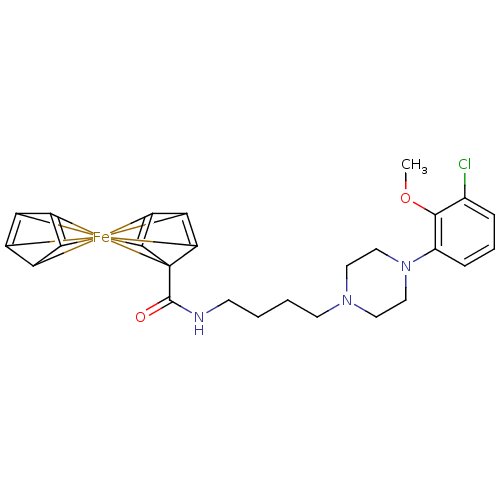 (CHEMBL195083 | N-{4-[4-(3-chloro-2-methoxyphenyl)p...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to human dopamine receptor D4.4 expressed in Chinese hamster ovary cells | J Med Chem 48: 3696-9 (2005) Article DOI: 10.1021/jm050170s BindingDB Entry DOI: 10.7270/Q20K29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50167935 (CHEMBL195083 | N-{4-[4-(3-chloro-2-methoxyphenyl)p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to human dopamine receptor D3 expressed in Chinese hamster ovary cells | J Med Chem 48: 3696-9 (2005) Article DOI: 10.1021/jm050170s BindingDB Entry DOI: 10.7270/Q20K29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50167935 (CHEMBL195083 | N-{4-[4-(3-chloro-2-methoxyphenyl)p...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Binding affinity towards porcine serotonin receptor 5-HT1A using [3H]8-OH-DPAT | J Med Chem 48: 3696-9 (2005) Article DOI: 10.1021/jm050170s BindingDB Entry DOI: 10.7270/Q20K29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50167935 (CHEMBL195083 | N-{4-[4-(3-chloro-2-methoxyphenyl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to human dopamine receptor D2 short expressed in Chinese hamster ovary cells | J Med Chem 48: 3696-9 (2005) Article DOI: 10.1021/jm050170s BindingDB Entry DOI: 10.7270/Q20K29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50167935 (CHEMBL195083 | N-{4-[4-(3-chloro-2-methoxyphenyl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to human dopamine receptor D2 long expressed in Chinese hamster ovary cells | J Med Chem 48: 3696-9 (2005) Article DOI: 10.1021/jm050170s BindingDB Entry DOI: 10.7270/Q20K29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine D1 receptor (Sus scrofa) | BDBM50167935 (CHEMBL195083 | N-{4-[4-(3-chloro-2-methoxyphenyl)p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Inhibition of [3H]-SCH- 23390 binding to dopamine receptor D1 of porcine striatal membranes | J Med Chem 48: 3696-9 (2005) Article DOI: 10.1021/jm050170s BindingDB Entry DOI: 10.7270/Q20K29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50167935 (CHEMBL195083 | N-{4-[4-(3-chloro-2-methoxyphenyl)p...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Effective concentration for [35S]GTP-gamma-S, binding in CHO-K1 cells expressing human dopamine D4 receptor | J Med Chem 48: 3696-9 (2005) Article DOI: 10.1021/jm050170s BindingDB Entry DOI: 10.7270/Q20K29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50167935 (CHEMBL195083 | N-{4-[4-(3-chloro-2-methoxyphenyl)p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Effective concentration in [3H]thymidine uptake assay by CHO dhfr- mutant cells expressing human D3 receptor | J Med Chem 48: 3696-9 (2005) Article DOI: 10.1021/jm050170s BindingDB Entry DOI: 10.7270/Q20K29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50167935 (CHEMBL195083 | N-{4-[4-(3-chloro-2-methoxyphenyl)p...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Effective concentration from [3H]thymidine uptake assay in CHO10001 cells expressing human Dopamine receptor D4.2 | J Med Chem 48: 3696-9 (2005) Article DOI: 10.1021/jm050170s BindingDB Entry DOI: 10.7270/Q20K29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||