

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50168965 (3S,4aS,6S,8aR)-6-[2-(1H-Tetrazol-5-yl)-phenoxy]-decahydro-isoquinoline-3-carboxylic acid::CHEMBL189985
SMILES: OC(=O)[C@@H]1C[C@H]2C[C@H](CC[C@H]2CN1)Oc1ccccc1-c1nnn[nH]1
InChI Key: InChIKey=MBVYDDACFKHYKD-OPDFLTKYSA-N
Data: 5 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor ionotropic kainate (Homo sapiens (Human)) | BDBM50168965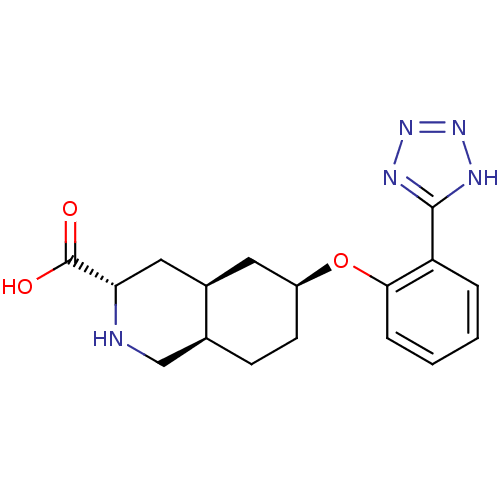 ((3S,4aS,6S,8aR)-6-[2-(1H-Tetrazol-5-yl)-phenoxy]-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]ATPA from human Gluk1 receptor | Bioorg Med Chem Lett 23: 6463-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.045 BindingDB Entry DOI: 10.7270/Q2XS5ZBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GRIK1 (Homo sapiens (Human)) | BDBM50168965 ((3S,4aS,6S,8aR)-6-[2-(1H-Tetrazol-5-yl)-phenoxy]-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigación Lilly Curated by ChEMBL | Assay Description Inhibition of [3H]-KA binding to iontropic glutamate receptor 5 | J Med Chem 48: 4200-3 (2005) Article DOI: 10.1021/jm0491952 BindingDB Entry DOI: 10.7270/Q2HQ3ZDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GRIA2 (Homo sapiens (Human)) | BDBM50168965 ((3S,4aS,6S,8aR)-6-[2-(1H-Tetrazol-5-yl)-phenoxy]-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigación Lilly Curated by ChEMBL | Assay Description Inhibition of [3H]-AMPA binding to human GluR2 receptors expressed in HEK 293 cells | J Med Chem 48: 4200-3 (2005) Article DOI: 10.1021/jm0491952 BindingDB Entry DOI: 10.7270/Q2HQ3ZDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor AMPA 1/2 (Homo sapiens (Human)) | BDBM50168965 ((3S,4aS,6S,8aR)-6-[2-(1H-Tetrazol-5-yl)-phenoxy]-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from homomeric recombinant GluA2 receptor (unknown origin) | Bioorg Med Chem Lett 23: 6463-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.045 BindingDB Entry DOI: 10.7270/Q2XS5ZBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM50168965 ((3S,4aS,6S,8aR)-6-[2-(1H-Tetrazol-5-yl)-phenoxy]-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigación Lilly Curated by ChEMBL | Assay Description Inhibition of [3H]-KA binding to iontropic glutamate receptor 6 | J Med Chem 48: 4200-3 (2005) Article DOI: 10.1021/jm0491952 BindingDB Entry DOI: 10.7270/Q2HQ3ZDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||