

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: O[C@@H]([C@@H](NC[C@H]1NC[C@H](O)[C@@H]1O)c1ccccc1)c1ccccc1
InChI Key: InChIKey=MAKKYIXPCPJUFC-ICBNADEASA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| alpha-1,2-Mannosidase (Glycine max) | BDBM50168990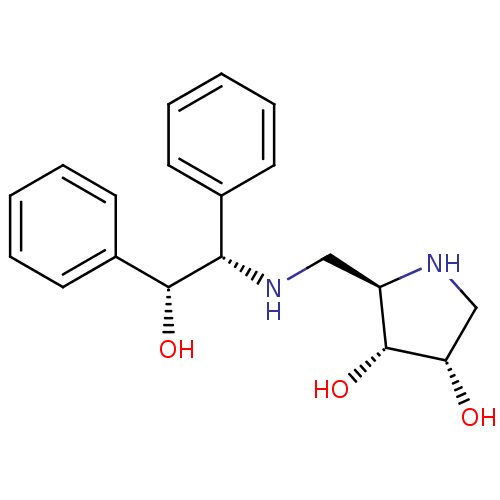 ((2R,3R,4S)-2-[((R)-Phenyl-1-(S)-2-hydroxy-2-phenyl...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Sciences and Engineering Curated by ChEMBL | Assay Description Concentration of compound inhibiting alpha-Mannosidase isolated from Jack bean | J Med Chem 48: 4237-46 (2005) Article DOI: 10.1021/jm0409019 BindingDB Entry DOI: 10.7270/Q24J0DNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endoplasmic reticulum mannosyl-oligosaccharide 1,2-alpha-mannosidase (Homo sapiens (Human)) | BDBM50168990 ((2R,3R,4S)-2-[((R)-Phenyl-1-(S)-2-hydroxy-2-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687 Curated by ChEMBL | Assay Description Inhibition of human ER alpha mannosidase 1 | Bioorg Med Chem 20: 6945-59 (2012) Article DOI: 10.1016/j.bmc.2012.10.011 BindingDB Entry DOI: 10.7270/Q26T0NTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Homo sapiens (Human)) | BDBM50168990 ((2R,3R,4S)-2-[((R)-Phenyl-1-(S)-2-hydroxy-2-phenyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687 Curated by ChEMBL | Assay Description Inhibition of human golgi alpha mannosidase 2 | Bioorg Med Chem 20: 6945-59 (2012) Article DOI: 10.1016/j.bmc.2012.10.011 BindingDB Entry DOI: 10.7270/Q26T0NTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||