

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50169351 CHEMBL3805852
SMILES: COc1cnc(cn1)C(=O)Nc1cc(F)c(F)c(c1)[C@]1(C)C[C@H](SC(N)=N1)c1c(C)noc1C
InChI Key: InChIKey=IFMZPRCGPCQBAP-AOMKIAJQSA-N
Data: 10 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM50169351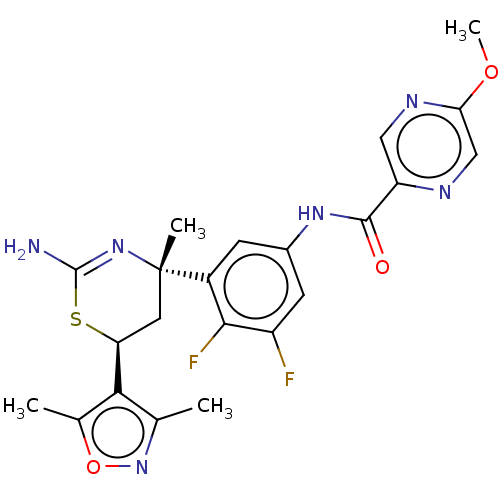 (CHEMBL3805852) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) expressed in baculovirus infected insect cells using CEC as susbtrate incubated for 45 mins by plate reader ana... | ACS Med Chem Lett 7: 271-6 (2016) BindingDB Entry DOI: 10.7270/Q2NS0WTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50169351 (CHEMBL3805852) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) expressed in baculovirus infected insect cells using MFC as susbtrate incubated for 45 mins by plate reader ana... | ACS Med Chem Lett 7: 271-6 (2016) BindingDB Entry DOI: 10.7270/Q2NS0WTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50169351 (CHEMBL3805852) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) expressed in baculovirus infected insect cells using CEC as susbtrate incubated for 45 mins by plate reader an... | ACS Med Chem Lett 7: 271-6 (2016) BindingDB Entry DOI: 10.7270/Q2NS0WTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50169351 (CHEMBL3805852) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) expressed in baculovirus infected insect cells using AMMC as susbtrate incubated for 45 mins by plate reader an... | ACS Med Chem Lett 7: 271-6 (2016) BindingDB Entry DOI: 10.7270/Q2NS0WTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50169351 (CHEMBL3805852) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Bioorg Med Chem Lett 29: 761-777 (2019) Article DOI: 10.1016/j.bmcl.2018.12.049 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50169351 (CHEMBL3805852) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) expressed in baculovirus infected insect cells using benzyloxyresorufin as susbtrate incubated for 45 mins by p... | ACS Med Chem Lett 7: 271-6 (2016) BindingDB Entry DOI: 10.7270/Q2NS0WTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50169351 (CHEMBL3805852) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of CYP2C8 (unknown origin) | ACS Med Chem Lett 7: 271-6 (2016) BindingDB Entry DOI: 10.7270/Q2NS0WTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50169351 (CHEMBL3805852) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing APP751 Swedish mutant assessed as inhibition of amyloid beta 40 or amyloid beta 42 production incuba... | ACS Med Chem Lett 7: 271-6 (2016) BindingDB Entry DOI: 10.7270/Q2NS0WTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50169351 (CHEMBL3805852) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human ERG by whole-cell patch clamp assay | ACS Med Chem Lett 7: 271-6 (2016) BindingDB Entry DOI: 10.7270/Q2NS0WTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50169351 (CHEMBL3805852) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) expressed in baculovirus infected insect cells using BFC as susbtrate incubated for 45 mins by plate reader ana... | ACS Med Chem Lett 7: 271-6 (2016) BindingDB Entry DOI: 10.7270/Q2NS0WTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||