

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50170888 CHEMBL3805557
SMILES: CCCNCC(N)P(O)(O)=O
InChI Key: InChIKey=WQIIZRIJSQZVGR-UHFFFAOYSA-N
Data: 4 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50170888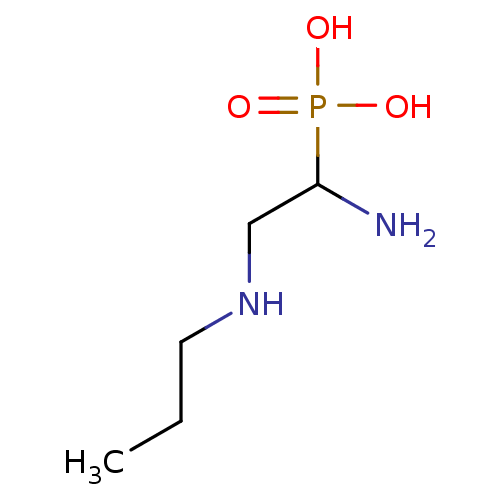 (CHEMBL3805557) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human alanyl aminopeptidase M1 using Ala-AMC as substrate preincubated for 30 to 60 mins followed by substrate addition mea... | Eur J Med Chem 117: 187-96 (2016) BindingDB Entry DOI: 10.7270/Q2BC41F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50170888 (CHEMBL3805557) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Inhibition of pig kidney alanyl aminopeptidase M1 using L-leucine p-nitro-anilide as substrate by spectrophotometric analysis | Eur J Med Chem 117: 187-96 (2016) BindingDB Entry DOI: 10.7270/Q2BC41F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endoplasmic reticulum aminopeptidase 2 (Homo sapiens (Human)) | BDBM50170888 (CHEMBL3805557) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Inhibition of human ERAP2 preincubated for 30 to 60 mins followed by addition of Arg-AMC as substrate measured for 15 mins by spectrofluorimetric met... | Bioorg Med Chem Lett 26: 4122-6 (2016) Article DOI: 10.1016/j.bmcl.2016.06.062 BindingDB Entry DOI: 10.7270/Q2TT4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endoplasmic reticulum aminopeptidase 1 (Homo sapiens (Human)) | BDBM50170888 (CHEMBL3805557) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human ERAP1 using L-AMC as substrate measured for 15 to 30 mins by fluorescence analysis | Bioorg Med Chem Lett 26: 4122-6 (2016) Article DOI: 10.1016/j.bmcl.2016.06.062 BindingDB Entry DOI: 10.7270/Q2TT4SW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||