

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50172459 1-(2-Fluoro-4''-hydroxy-[1,1';4',1'']terphenyl-4-yl)-cyclopropanecarboxylic acid::CHEMBL196947
SMILES: OC(=O)C1(CC1)c1ccc(c(F)c1)-c1ccc(cc1)-c1ccc(O)cc1
InChI Key: InChIKey=TUOLQOHKRDLKLU-UHFFFAOYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta amyloid A4 protein (Homo sapiens (Human)) | BDBM50172459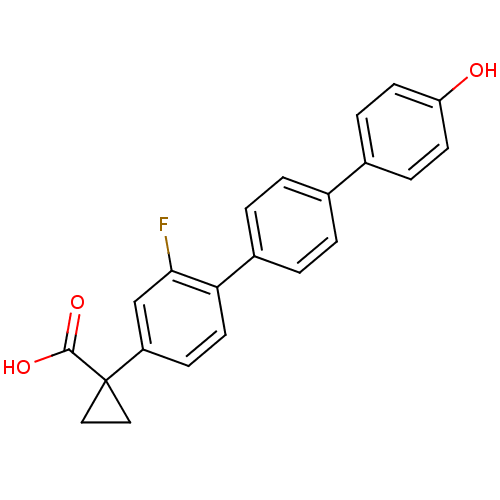 (1-(2-Fluoro-4''-hydroxy-[1,1';4',1'']terphenyl-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiesi Farmaceutici S.p.A. Curated by ChEMBL | Assay Description Concentration required to inhibit A beta 40 peptide 100 uM | J Med Chem 48: 5705-20 (2005) Article DOI: 10.1021/jm0502541 BindingDB Entry DOI: 10.7270/Q2CC106W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta amyloid A4 protein (Homo sapiens (Human)) | BDBM50172459 (1-(2-Fluoro-4''-hydroxy-[1,1';4',1'']terphenyl-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiesi Farmaceutici S.p.A. Curated by ChEMBL | Assay Description Inhibitory concentration against beta-amyloid-42 (Abeta42) secretion was evaluated in human neuroglioma cells (H4-APP695NL) | J Med Chem 48: 5705-20 (2005) Article DOI: 10.1021/jm0502541 BindingDB Entry DOI: 10.7270/Q2CC106W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50172459 (1-(2-Fluoro-4''-hydroxy-[1,1';4',1'']terphenyl-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiesi Farmaceutici S.p.A. Curated by ChEMBL | Assay Description Interaction with human cytochrome P450 isoform 2C19 expressed in baculovirus-insect cells | J Med Chem 48: 5705-20 (2005) Article DOI: 10.1021/jm0502541 BindingDB Entry DOI: 10.7270/Q2CC106W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50172459 (1-(2-Fluoro-4''-hydroxy-[1,1';4',1'']terphenyl-4-y...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiesi Farmaceutici S.p.A. Curated by ChEMBL | Assay Description Interaction with human cytochrome P450 isoform 2D6 expressed in baculovirus-insect cells | J Med Chem 48: 5705-20 (2005) Article DOI: 10.1021/jm0502541 BindingDB Entry DOI: 10.7270/Q2CC106W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50172459 (1-(2-Fluoro-4''-hydroxy-[1,1';4',1'']terphenyl-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiesi Farmaceutici S.p.A. Curated by ChEMBL | Assay Description Interaction with human cytochrome P450 isoform 2C9 expressed in baculovirus-insect cells | J Med Chem 48: 5705-20 (2005) Article DOI: 10.1021/jm0502541 BindingDB Entry DOI: 10.7270/Q2CC106W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||