 Found 7 hits for monomerid = 50172473
Found 7 hits for monomerid = 50172473 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50172473
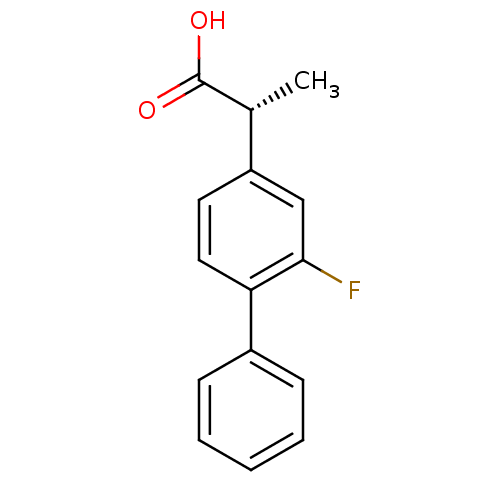
((-)-(2R)-2-(2-fluorobiphenyl-4-yl)propanoic acid |...)Show InChI InChI=1S/C15H13FO2/c1-10(15(17)18)12-7-8-13(14(16)9-12)11-5-3-2-4-6-11/h2-10H,1H3,(H,17,18)/t10-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in human whole blood |
Bioorg Med Chem Lett 16: 2219-23 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.033
BindingDB Entry DOI: 10.7270/Q2M32VC8 |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50172473

((-)-(2R)-2-(2-fluorobiphenyl-4-yl)propanoic acid |...)Show InChI InChI=1S/C15H13FO2/c1-10(15(17)18)12-7-8-13(14(16)9-12)11-5-3-2-4-6-11/h2-10H,1H3,(H,17,18)/t10-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of CHAPSO-solubilized gamma-secretase isolated from human SH-SY5Y P2 cell membranes assessed as reduction in Abeta(1 to 42) peptide genera... |
Bioorg Med Chem Lett 25: 841-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.073
BindingDB Entry DOI: 10.7270/Q2280989 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50172473

((-)-(2R)-2-(2-fluorobiphenyl-4-yl)propanoic acid |...)Show InChI InChI=1S/C15H13FO2/c1-10(15(17)18)12-7-8-13(14(16)9-12)11-5-3-2-4-6-11/h2-10H,1H3,(H,17,18)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiesi Farmaceutici S.p.A.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against beta-amyloid-42 (Abeta42) secretion was evaluated in human neuroglioma cells (H4-APP695NL) |
J Med Chem 48: 5705-20 (2005)
Article DOI: 10.1021/jm0502541
BindingDB Entry DOI: 10.7270/Q2CC106W |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50172473

((-)-(2R)-2-(2-fluorobiphenyl-4-yl)propanoic acid |...)Show InChI InChI=1S/C15H13FO2/c1-10(15(17)18)12-7-8-13(14(16)9-12)11-5-3-2-4-6-11/h2-10H,1H3,(H,17,18)/t10-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV)
Curated by ChEMBL
| Assay Description
Modulation of gamma secretase (unknown origin) assessed as reduction in amyloid beta levels |
J Med Chem 59: 7719-37 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01516 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50172473

((-)-(2R)-2-(2-fluorobiphenyl-4-yl)propanoic acid |...)Show InChI InChI=1S/C15H13FO2/c1-10(15(17)18)12-7-8-13(14(16)9-12)11-5-3-2-4-6-11/h2-10H,1H3,(H,17,18)/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.23E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiesi Farmaceutici S.p.A.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit cyclooxygenase-2 in human blood |
J Med Chem 48: 5705-20 (2005)
Article DOI: 10.1021/jm0502541
BindingDB Entry DOI: 10.7270/Q2CC106W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50172473

((-)-(2R)-2-(2-fluorobiphenyl-4-yl)propanoic acid |...)Show InChI InChI=1S/C15H13FO2/c1-10(15(17)18)12-7-8-13(14(16)9-12)11-5-3-2-4-6-11/h2-10H,1H3,(H,17,18)/t10-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated 2-arachidonoylglycerol oxygenation in LPS/IFN-gamma-stimulated mouse RAW264.7 cells assessed as 2-AG to PGE2-G/PGD2-G con... |
ACS Med Chem Lett 3: 759-763 (2012)
Article DOI: 10.1021/ml3001616
BindingDB Entry DOI: 10.7270/Q2FJ2HWS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(RAT) | BDBM50172473

((-)-(2R)-2-(2-fluorobiphenyl-4-yl)propanoic acid |...)Show InChI InChI=1S/C15H13FO2/c1-10(15(17)18)12-7-8-13(14(16)9-12)11-5-3-2-4-6-11/h2-10H,1H3,(H,17,18)/t10-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiesi Farmaceutici S.p.A.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit cyclooxygenase-1 in rat blood |
J Med Chem 48: 5705-20 (2005)
Article DOI: 10.1021/jm0502541
BindingDB Entry DOI: 10.7270/Q2CC106W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data