

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50173540 1-[5-(tert-Butyl-diphenyl-silanyloxymethyl)-2,5-dihydro-furan-2-yl]-1H-pyrimidine-2,4-dione::CHEMBL371690::dUTPase inhibitor, 5
SMILES: CC(C)(C)[Si](OCC1OC(C=C1)n1ccc(=O)[nH]c1=O)(c1ccccc1)c1ccccc1
InChI Key: InChIKey=IHZUQZWWKIYBME-UHFFFAOYSA-N
Data: 6 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dUTP pyrophosphatase (Plasmodium falciparum) | BDBM50173540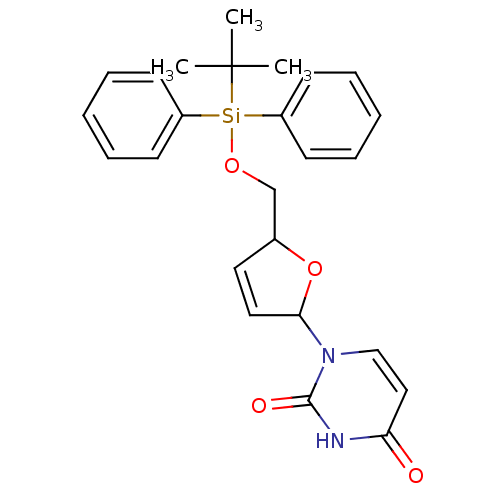 (1-[5-(tert-Butyl-diphenyl-silanyloxymethyl)-2,5-di...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.16E+3 | -8.09 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Instituto de Parastiologia y Biomedicina "Lopez-Neyra" | Assay Description Nucleotide hydrolysis was monitored by mixing enzyme and substrate with a rapid kinetic accessory (Hi-Tech Scientific) attached to a spectrophotomete... | J Enzyme Inhib Med Chem 24: 111-6 (2009) Article DOI: 10.1080/14756360801915476 BindingDB Entry DOI: 10.7270/Q2JH3JST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dUTP pyrophosphatase (Plasmodium falciparum) | BDBM50173540 (1-[5-(tert-Butyl-diphenyl-silanyloxymethyl)-2,5-di...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibitory constant against Plasmodium falciparum deoxyuridine 5'-triphosphate nucleotidohydrolase expressed in Escherichia coli BL21 (DE3) cells | J Med Chem 48: 5942-54 (2005) Article DOI: 10.1021/jm050111e BindingDB Entry DOI: 10.7270/Q2Z320DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| dUTP pyrophosphatase (Leishmania major) | BDBM50173540 (1-[5-(tert-Butyl-diphenyl-silanyloxymethyl)-2,5-di...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibitory constant against Leishmania major deoxyuridine 5'-triphosphate nucleotidohydrolase | J Med Chem 48: 5942-54 (2005) Article DOI: 10.1021/jm050111e BindingDB Entry DOI: 10.7270/Q2Z320DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (dUTPase) (Homo sapiens (Human)) | BDBM50173540 (1-[5-(tert-Butyl-diphenyl-silanyloxymethyl)-2,5-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibitory constant against human deoxyuridine 5'-triphosphate nucleotidohydrolase expressed in Escherichia coli BL21 (DE3) cells | J Med Chem 48: 5942-54 (2005) Article DOI: 10.1021/jm050111e BindingDB Entry DOI: 10.7270/Q2Z320DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (dUTPase) (Homo sapiens (Human)) | BDBM50173540 (1-[5-(tert-Butyl-diphenyl-silanyloxymethyl)-2,5-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+6 | >-4.09 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Instituto de Parastiologia y Biomedicina "Lopez-Neyra" | Assay Description Nucleotide hydrolysis was monitored by mixing enzyme and substrate with a rapid kinetic accessory (Hi-Tech Scientific) attached to a spectrophotomete... | J Enzyme Inhib Med Chem 24: 111-6 (2009) Article DOI: 10.1080/14756360801915476 BindingDB Entry DOI: 10.7270/Q2JH3JST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (dUTPase) (Campylobacter jejuni) | BDBM50173540 (1-[5-(tert-Butyl-diphenyl-silanyloxymethyl)-2,5-di...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+9 | 0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Instituto de Parastiologia y Biomedicina "Lopez-Neyra" | Assay Description Nucleotide hydrolysis was monitored by mixing enzyme and substrate with a rapid kinetic accessory (Hi-Tech Scientific) attached to a spectrophotomete... | J Enzyme Inhib Med Chem 24: 111-6 (2009) Article DOI: 10.1080/14756360801915476 BindingDB Entry DOI: 10.7270/Q2JH3JST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||