

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50177332 CHEMBL199472::potassium O-(2-(heptadec-9-enyl)-1,3-dioxolan-4-yl)methyl O-hydrogenphosphorothioate
SMILES: CCCCCCC\C=C\CCCCCCCCC1OCC(COP(O)([O-])=S)O1
InChI Key: InChIKey=GPCDCYBCUTWZFV-CMDGGOBGSA-M
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50177332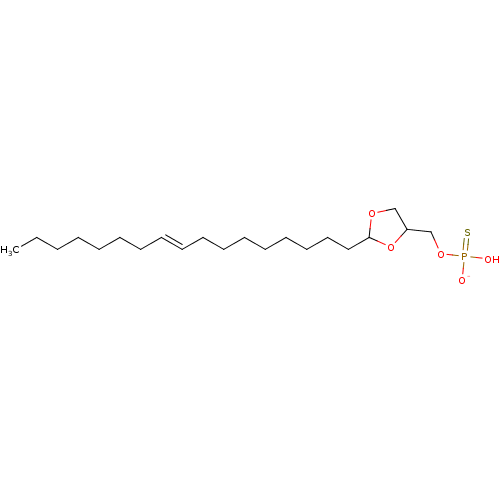 (CHEMBL199472 | potassium O-(2-(heptadec-9-enyl)-1,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 415 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Inhibition of lysophospholipase D autotaxin | Bioorg Med Chem Lett 16: 451-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.096 BindingDB Entry DOI: 10.7270/Q2Z89C01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor Edg-4 (Homo sapiens (Human)) | BDBM50177332 (CHEMBL199472 | potassium O-(2-(heptadec-9-enyl)-1,...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA2 receptor in RH7777 rat hepatoma cell line | Bioorg Med Chem Lett 16: 451-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.096 BindingDB Entry DOI: 10.7270/Q2Z89C01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1/lysophosphatidic acid receptor 3 (Rattus norvegicus) | BDBM50177332 (CHEMBL199472 | potassium O-(2-(heptadec-9-enyl)-1,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 639 | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA3 receptor in RH7777 rat hepatoma cell line | Bioorg Med Chem Lett 16: 451-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.096 BindingDB Entry DOI: 10.7270/Q2Z89C01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1/lysophosphatidic acid receptor 3 (Rattus norvegicus) | BDBM50177332 (CHEMBL199472 | potassium O-(2-(heptadec-9-enyl)-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 981 | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA1 receptor in RH7777 rat hepatoma cell line | Bioorg Med Chem Lett 16: 451-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.096 BindingDB Entry DOI: 10.7270/Q2Z89C01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||