

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50184782 CHEMBL3824228::US10294227, Code 543
SMILES: CN(C)C1CCN(CCn2nc(-c3ccc(NC(=O)OC(C)(C)C)cc3)c3c(N)ncnc23)CC1
InChI Key: InChIKey=DPZGKYJHTOSMIA-UHFFFAOYSA-N
Data: 17 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50184782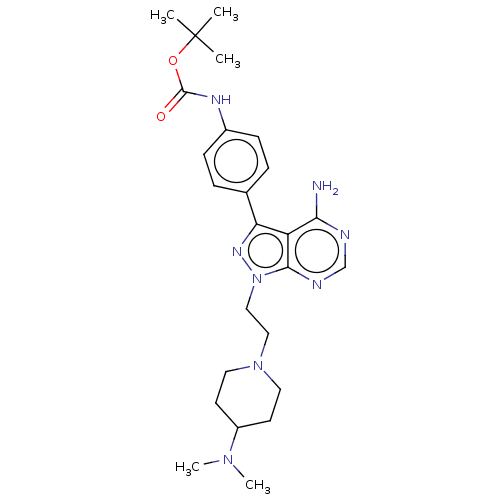 (CHEMBL3824228 | US10294227, Code 543) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh Curated by ChEMBL | Assay Description Inhibition of human ABL using EAIYAAPFAKKK as substrate in presence of [gamma-33P]ATP | J Med Chem 59: 4697-710 (2016) BindingDB Entry DOI: 10.7270/Q2B85B2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM50184782 (CHEMBL3824228 | US10294227, Code 543) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction... | Bioorg Med Chem Lett 19: 773-7 (2009) BindingDB Entry DOI: 10.7270/Q27D2XFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50184782 (CHEMBL3824228 | US10294227, Code 543) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction... | Bioorg Med Chem Lett 19: 773-7 (2009) BindingDB Entry DOI: 10.7270/Q27D2XFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Blk (Homo sapiens (Human)) | BDBM50184782 (CHEMBL3824228 | US10294227, Code 543) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction... | Bioorg Med Chem Lett 19: 773-7 (2009) BindingDB Entry DOI: 10.7270/Q27D2XFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BRK (Homo sapiens (Human)) | BDBM50184782 (CHEMBL3824228 | US10294227, Code 543) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction... | Bioorg Med Chem Lett 19: 773-7 (2009) BindingDB Entry DOI: 10.7270/Q27D2XFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fgr (Homo sapiens (Human)) | BDBM50184782 (CHEMBL3824228 | US10294227, Code 543) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction... | Bioorg Med Chem Lett 19: 773-7 (2009) BindingDB Entry DOI: 10.7270/Q27D2XFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase FRK (Homo sapiens (Human)) | BDBM50184782 (CHEMBL3824228 | US10294227, Code 543) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction... | Bioorg Med Chem Lett 19: 773-7 (2009) BindingDB Entry DOI: 10.7270/Q27D2XFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50184782 (CHEMBL3824228 | US10294227, Code 543) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction... | Bioorg Med Chem Lett 19: 773-7 (2009) BindingDB Entry DOI: 10.7270/Q27D2XFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM50184782 (CHEMBL3824228 | US10294227, Code 543) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction... | Bioorg Med Chem Lett 19: 773-7 (2009) BindingDB Entry DOI: 10.7270/Q27D2XFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50184782 (CHEMBL3824228 | US10294227, Code 543) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction... | Bioorg Med Chem Lett 19: 773-7 (2009) BindingDB Entry DOI: 10.7270/Q27D2XFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50184782 (CHEMBL3824228 | US10294227, Code 543) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction... | Bioorg Med Chem Lett 19: 773-7 (2009) BindingDB Entry DOI: 10.7270/Q27D2XFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM50184782 (CHEMBL3824228 | US10294227, Code 543) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction... | Bioorg Med Chem Lett 19: 773-7 (2009) BindingDB Entry DOI: 10.7270/Q27D2XFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50184782 (CHEMBL3824228 | US10294227, Code 543) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction... | Bioorg Med Chem Lett 19: 773-7 (2009) BindingDB Entry DOI: 10.7270/Q27D2XFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50184782 (CHEMBL3824228 | US10294227, Code 543) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction... | Bioorg Med Chem Lett 19: 773-7 (2009) BindingDB Entry DOI: 10.7270/Q27D2XFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50184782 (CHEMBL3824228 | US10294227, Code 543) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction... | Bioorg Med Chem Lett 19: 773-7 (2009) BindingDB Entry DOI: 10.7270/Q27D2XFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50184782 (CHEMBL3824228 | US10294227, Code 543) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Compound IC50 values were determined from 10-point, 1:3 dilution curves starting at either 100 μM or 10 μM with 10 μM ATP, by Reaction... | Bioorg Med Chem Lett 19: 773-7 (2009) BindingDB Entry DOI: 10.7270/Q27D2XFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50184782 (CHEMBL3824228 | US10294227, Code 543) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged full length human SRC expressed in insect cells preincubated for 20 mins using poly[Glu,Tyr]4:1 as substrate meas... | J Med Chem 59: 4697-710 (2016) BindingDB Entry DOI: 10.7270/Q2B85B2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||