

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50184823 Ac-Lys(N-epsilon-biotinoyl)-Ala-Ala-Bip-Thr(PO3H2)-Pro-Nal-Gln-NH2::CHEMBL438271
SMILES: C[C@H](NC(=O)[C@H](CCCCNC(=O)CCCC[C@@H]1SC[C@@H]2NC(=O)N[C@H]12)NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N[C@@H]([C@H](C)OP(O)(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](CCC(N)=O)C(N)=O
InChI Key: InChIKey=KTBHFKXUIQPWNG-IQMYXQEDSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50184823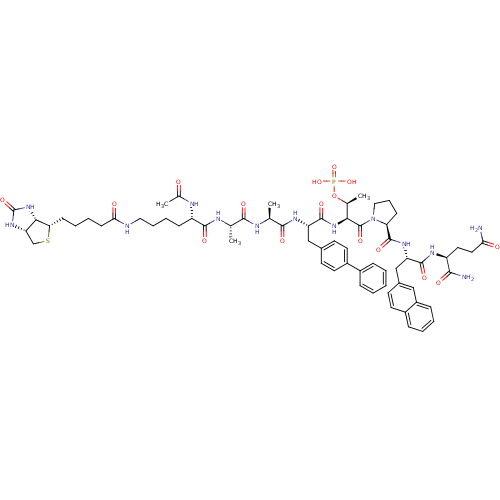 (Ac-Lys(N-epsilon-biotinoyl)-Ala-Ala-Bip-Thr(PO3H2)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of human Pin1 PPIase Activity by protease free PPIase assay | J Med Chem 49: 2147-50 (2006) Article DOI: 10.1021/jm060036n BindingDB Entry DOI: 10.7270/Q2S46RJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50184823 (Ac-Lys(N-epsilon-biotinoyl)-Ala-Ala-Bip-Thr(PO3H2)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of human Pin1 PPIase Activity by protease free PPIase assay | J Med Chem 49: 2147-50 (2006) Article DOI: 10.1021/jm060036n BindingDB Entry DOI: 10.7270/Q2S46RJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||