 Found 3 hits for monomerid = 50189746
Found 3 hits for monomerid = 50189746 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50189746
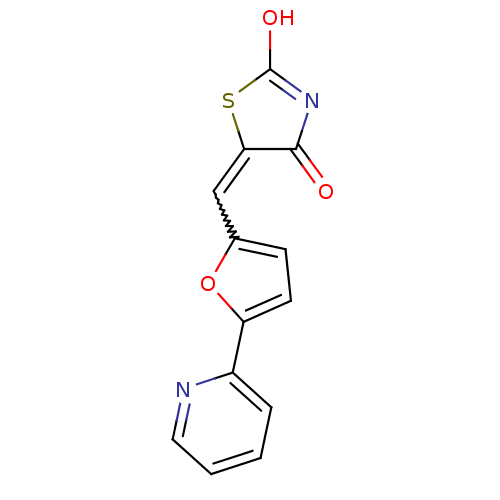
(5-(5-pyridin-2-ylfuran-2-ylmethylene)thiazolidine-...)Show SMILES OC1=NC(=O)C(S1)=Cc1ccc(o1)-c1ccccn1 |w:7.8,t:1| Show InChI InChI=1S/C13H8N2O3S/c16-12-11(19-13(17)15-12)7-8-4-5-10(18-8)9-3-1-2-6-14-9/h1-7H,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) assessed as decrease in fluorescence intensity using phosphorylated substrate |
Eur J Med Chem 84: 454-65 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.056
BindingDB Entry DOI: 10.7270/Q2057HM8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50189746

(5-(5-pyridin-2-ylfuran-2-ylmethylene)thiazolidine-...)Show SMILES OC1=NC(=O)C(S1)=Cc1ccc(o1)-c1ccccn1 |w:7.8,t:1| Show InChI InChI=1S/C13H8N2O3S/c16-12-11(19-13(17)15-12)7-8-4-5-10(18-8)9-3-1-2-6-14-9/h1-7H,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma |
J Med Chem 49: 3857-71 (2006)
Article DOI: 10.1021/jm0601598
BindingDB Entry DOI: 10.7270/Q2DZ07X0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50189746

(5-(5-pyridin-2-ylfuran-2-ylmethylene)thiazolidine-...)Show SMILES OC1=NC(=O)C(S1)=Cc1ccc(o1)-c1ccccn1 |w:7.8,t:1| Show InChI InChI=1S/C13H8N2O3S/c16-12-11(19-13(17)15-12)7-8-4-5-10(18-8)9-3-1-2-6-14-9/h1-7H,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 185 | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha |
J Med Chem 49: 3857-71 (2006)
Article DOI: 10.1021/jm0601598
BindingDB Entry DOI: 10.7270/Q2DZ07X0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data