

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50191436 (2'Z,3'E)-7-bromoindirubin-3'-[O-(2-piperazin-1-ylethyl)oxime] dihydrochloride::CHEMBL379713
SMILES: Oc1[nH]c2c(Br)cccc2c1C1=Nc2ccccc2\C1=N/OCC[NH+]1CC[NH2+]CC1
InChI Key: InChIKey=GKPYOIONIPTTMQ-NHFJDJAPSA-P
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50191436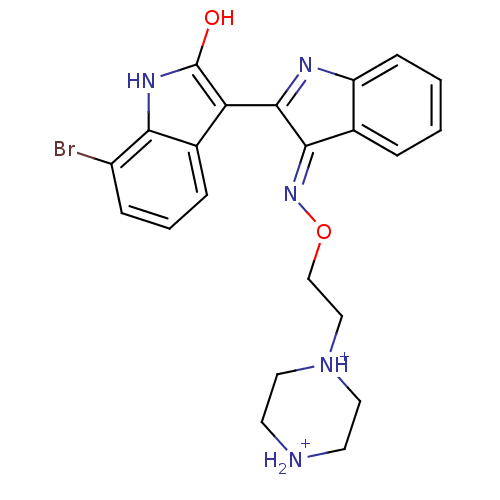 ((2'Z,3'E)-7-bromoindirubin-3'-[O-(2-piperazin-1-yl...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
C.N.R.S. Curated by ChEMBL | Assay Description Survival of mouse AhR +/+ 5L cells after 48 hrs by MTS reduction assay | J Med Chem 49: 4638-49 (2006) Article DOI: 10.1021/jm060314i BindingDB Entry DOI: 10.7270/Q21G0N3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-Dependent Kinase 5 (CDK5) (Homo sapiens (Human)) | BDBM50191436 ((2'Z,3'E)-7-bromoindirubin-3'-[O-(2-piperazin-1-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
C.N.R.S. Curated by ChEMBL | Assay Description Inhibition of mammalian CDK5/p25 | J Med Chem 49: 4638-49 (2006) Article DOI: 10.1021/jm060314i BindingDB Entry DOI: 10.7270/Q21G0N3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||