

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Cc1ncc(cc1NC(=O)c1cnn2cc(ccc12)-c1cnn(C)c1)C(=O)NCCN1CCCC1(C)C
InChI Key: InChIKey=WUPFLUOYXIDHAV-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50191978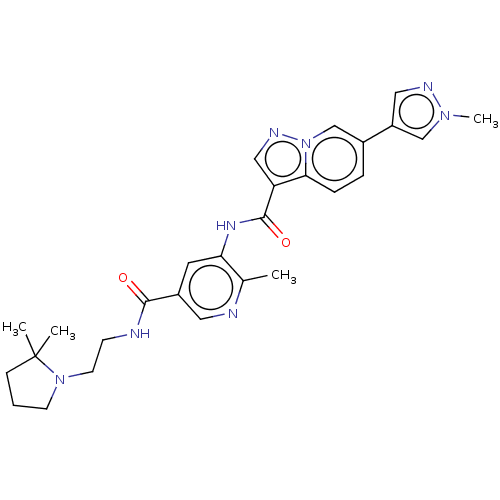 (CHEMBL3915941) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of H3 receptor (unknown origin) | J Med Chem 59: 7901-14 (2016) BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50191978 (CHEMBL3915941) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of M1 receptor (unknown origin) | J Med Chem 59: 7901-14 (2016) BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Rattus norvegicus) | BDBM50191978 (CHEMBL3915941) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFR-beta driven proliferation of rat A10 cells after 68 hrs in presence of rat recombinant PDGF-BB by cell titer-glo luminescence ass... | J Med Chem 59: 7901-14 (2016) BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GSC2 (Saccharomyces cerevisiae) | BDBM50191978 (CHEMBL3915941) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of human ERG | J Med Chem 59: 7901-14 (2016) BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191978 (CHEMBL3915941) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||