

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50192294 1-[(5R,6R,8R,9R)-4-amino-9-(tert-butyl-dimethyl-silanyloxy)-6-(tert-butyl-dimethyl-silanyloxymethyl)-3-(3-hydroxy-propenyl)-2,2-dioxo-1,7-dioxa-2lambda*6*-thia-spiro[4.4]non-3-en-8-yl]-5-methyl-1H-pyrimidine-2,4-dione::CHEMBL379255
SMILES: Cc1cn([C@@H]2O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@]3(OS(=O)(=O)C(=CCCO)C3=N)[C@H]2O[Si](C)(C)C(C)(C)C)c(=O)[nH]c1=O
InChI Key: InChIKey=PPSMYMVWZXRNAT-ZOZFQSSASA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50192294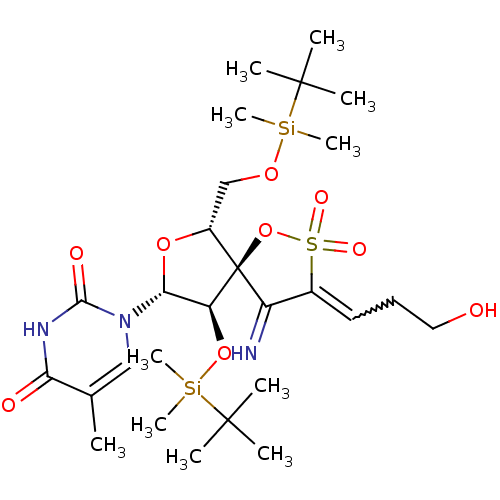 (1-[(5R,6R,8R,9R)-4-amino-9-(tert-butyl-dimethyl-si...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh School of Medicine Curated by ChEMBL | Assay Description Inhibition of FLAG-p66/HIS-p51-mediated HIV1 reverse transcriptase dimerization | J Med Chem 49: 4834-41 (2006) Article DOI: 10.1021/jm0604575 BindingDB Entry DOI: 10.7270/Q2GH9HKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50192294 (1-[(5R,6R,8R,9R)-4-amino-9-(tert-butyl-dimethyl-si...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (C.S.I.C.) Curated by ChEMBL | Assay Description Effective concentration required to inhibit HIV-1 induced cytopathicity in CEM cell culture by 50%. | J Med Chem 45: 3934-45 (2002) BindingDB Entry DOI: 10.7270/Q2639QXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50192294 (1-[(5R,6R,8R,9R)-4-amino-9-(tert-butyl-dimethyl-si...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (C.S.I.C.) Curated by ChEMBL | Assay Description Effective concentration required to inhibit HIV-1 induced cytopathicity in MT-4 cell culture by 50%. | J Med Chem 45: 3934-45 (2002) BindingDB Entry DOI: 10.7270/Q2639QXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50192294 (1-[(5R,6R,8R,9R)-4-amino-9-(tert-butyl-dimethyl-si...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh School of Medicine Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase RNA-dependent DNA polymerase activity | J Med Chem 49: 4834-41 (2006) Article DOI: 10.1021/jm0604575 BindingDB Entry DOI: 10.7270/Q2GH9HKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||