

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50192451 CHEMBL213053::NADP+
SMILES: NC(=O)c1ccc[n+](c1)C1OC(COP([O-])(=O)OP([O-])(=O)OC[C@H]2O[C@H]([C@H](OP([O-])([O-])=O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@H](O)[C@@H]1O
InChI Key: InChIKey=XJLXINKUBYWONI-CGXMUHRRSA-K
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate Reductase-Thymidylate Synthase (DHFR-TS) Mutant KICB1 (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50192451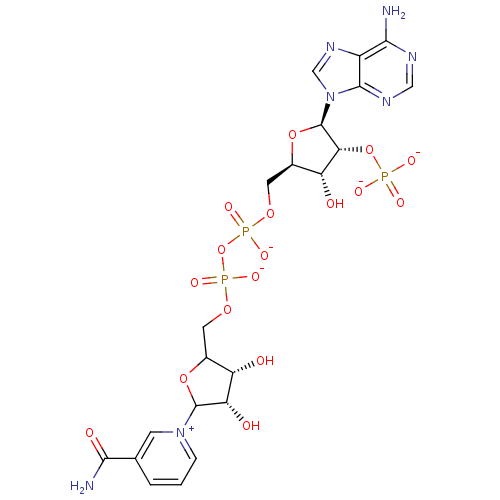 (CHEMBL213053 | NADP+) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Binding affinity was evaluated as inhibition of recombinant wild type (WT) Plasmodium falciparum DHFR-TS. | J Med Chem 41: 1367-70 (1998) Article DOI: 10.1021/jm970845u BindingDB Entry DOI: 10.7270/Q2ZC83JJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dihydrofolate Reductase-Thymidylate Synthase (DHFR-TS) Mutant KICB1 (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50192451 (CHEMBL213053 | NADP+) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Binding affinity was evaluated as inhibition of mutant (C59R + S108N) Plasmodium falciparum DHFR-TS. | J Med Chem 41: 1367-70 (1998) Article DOI: 10.1021/jm970845u BindingDB Entry DOI: 10.7270/Q2ZC83JJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Ketopantoate reductase (Escherichia coli (strain K12)) | BDBM50192451 (CHEMBL213053 | NADP+) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a |
University Chemical Laboratory Curated by ChEMBL | Assay Description Binding affinity to Escherichia coli KPR | J Med Chem 49: 4992-5000 (2006) Article DOI: 10.1021/jm060490r BindingDB Entry DOI: 10.7270/Q28S4QQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||