

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50192456 [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl ({[(3R,4S)-5-(3-carbamoyl-1,4-dihydropyridin-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl phosphonato}oxy)phosphonate
SMILES: NC(=O)C1=CN(C=CC1)C1OC(COP([O-])(=O)OP([O-])(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@H](O)[C@@H]1O
InChI Key: InChIKey=BOPGDPNILDQYTO-CGXMUHRRSA-L
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohol dehydrogenase (Homo sapiens (Human)) | BDBM50192456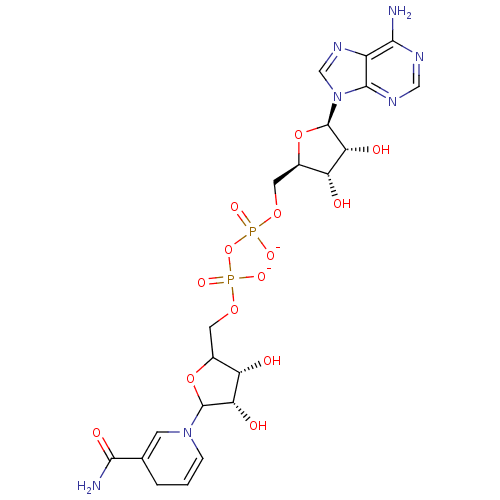 ([(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Medical Center Curated by ChEMBL | Assay Description Inhibition constant against mammalian liver alcohol dehydrogenase (ADH) | J Med Chem 37: 392-9 (1994) BindingDB Entry DOI: 10.7270/Q2RN38H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate dehydrogenase (Bos taurus) | BDBM50192456 ([(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester Medical Center Curated by ChEMBL | Assay Description Inhibition constant against mammalian glutamate dehydrogenase (GDH) | J Med Chem 37: 392-9 (1994) BindingDB Entry DOI: 10.7270/Q2RN38H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketopantoate reductase (Escherichia coli (strain K12)) | BDBM50192456 ([(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a |
University Chemical Laboratory Curated by ChEMBL | Assay Description Binding affinity to Escherichia coli KPR | J Med Chem 49: 4992-5000 (2006) Article DOI: 10.1021/jm060490r BindingDB Entry DOI: 10.7270/Q28S4QQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||