

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50193478 CID44415032::[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonatooxy)oxolan-2-yl]methyl {[(3R)-3-hydroxy-2,2-dimethyl-3-{[2-({2-[({[2-(piperazin-1-yl)ethyl]carbamoyl}methyl)sulfanyl]ethyl}carbamoyl)ethyl]carbamoyl}propyl phosphonato]oxy}phosphonate::truncated aminoglycoside-coenzyme A bisubstrate analogue 4e
SMILES: CC(C)(COP([O-])(=O)OP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP([O-])([O-])=O)n1cnc2c(N)ncnc12)[C@@H](O)C(=O)NCCC(=O)NCCSCC(=O)NCCN1CCNCC1
InChI Key: InChIKey=LYZBNZJJPFRRNF-CECATXLMSA-J
Data: 1 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminoglycoside acetyltransferase (Enterococcus durans) | BDBM50193478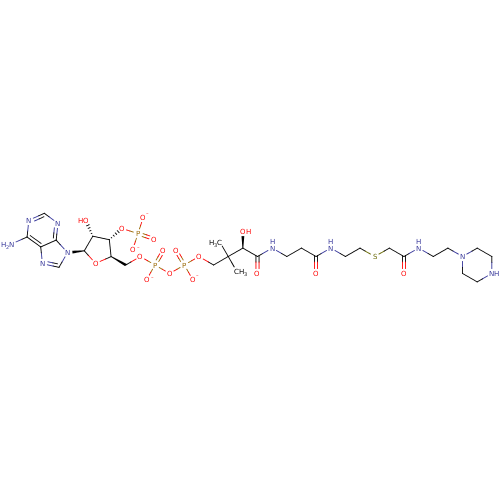 (CID44415032 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecium AAC(6')Ii | J Med Chem 49: 5273-81 (2006) Article DOI: 10.1021/jm060732n BindingDB Entry DOI: 10.7270/Q2183789 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||