

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: COC(=O)[C@H](CO)NC(=O)[C@H](CCCCN(C1CC2CCC1C2)C(C)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)c1ccccc1
InChI Key: InChIKey=STNKZTAUFWFKBE-YCIFDCPOSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chromobox protein homolog 5 (Homo sapiens (Human)) | BDBM50194252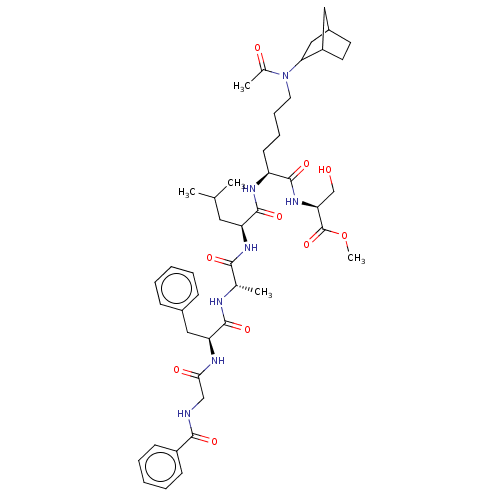 (CHEMBL3922982) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Antagonist activity at recombinant human N-terminal His-tagged CBX5 chromodomain (18 to 75 residues) expressed in Escherichia coli Rosetta BL21(DE3)p... | J Med Chem 59: 8913-8923 (2016) Article DOI: 10.1021/acs.jmedchem.6b00801 BindingDB Entry DOI: 10.7270/Q2H1340D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 SUMO-protein ligase CBX4 (Homo sapiens (Human)) | BDBM50194252 (CHEMBL3922982) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Antagonist activity at recombinant human N-terminal His-tagged CBX4 chromodomain (8 to 65 residues) expressed in Escherichia coli Rosetta BL21(DE3)pL... | J Med Chem 59: 8913-8923 (2016) Article DOI: 10.1021/acs.jmedchem.6b00801 BindingDB Entry DOI: 10.7270/Q2H1340D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chromodomain Y-like protein 2 (Homo sapiens (Human)) | BDBM50194252 (CHEMBL3922982) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Antagonist activity at recombinant human C-terminal His-tagged CDYL2 chromodomain (1 to 75 residues) expressed in Escherichia coli Rosetta BL21(DE3)p... | J Med Chem 59: 8913-8923 (2016) Article DOI: 10.1021/acs.jmedchem.6b00801 BindingDB Entry DOI: 10.7270/Q2H1340D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chromobox protein homolog 8 (Homo sapiens (Human)) | BDBM50194252 (CHEMBL3922982) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Antagonist activity at recombinant human N-terminal His-tagged CBX8 chromodomain (8 to 61 residues) expressed in Escherichia coli Rosetta BL21(DE3)pL... | J Med Chem 59: 8913-8923 (2016) Article DOI: 10.1021/acs.jmedchem.6b00801 BindingDB Entry DOI: 10.7270/Q2H1340D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chromobox protein homolog 7 (Homo sapiens (Human)) | BDBM50194252 (CHEMBL3922982) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Antagonist activity at recombinant human C-terminal His-tagged CBX7 chromodomain (8 to 62 residues) expressed in Escherichia coli Rosetta BL21(DE3)pL... | J Med Chem 59: 8913-8923 (2016) Article DOI: 10.1021/acs.jmedchem.6b00801 BindingDB Entry DOI: 10.7270/Q2H1340D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||