

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50195244 CHEMBL3926554::US10287258, Example 24::US10669245, Example 24
SMILES: Cn1c2cc(ccc2ccc1=O)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)cc1
InChI Key: InChIKey=BQIZWZAQEICNDG-GMAHTHKFSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase I (Homo sapiens (Human)) | BDBM50195244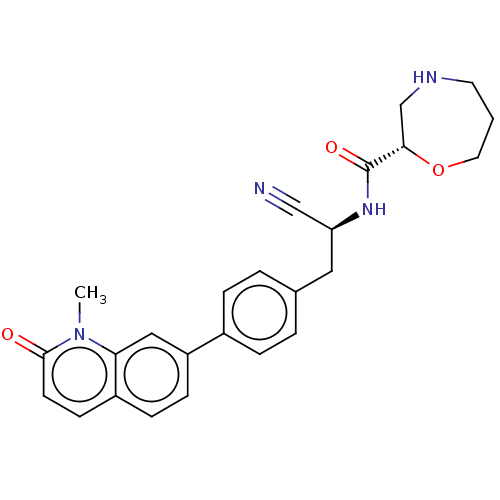 (CHEMBL3926554 | US10287258, Example 24 | US1066924...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
CHARLES RIVER DISCOVERY RESEARCH SERVICES UK LIMITED Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP1 using Gly-Arg-AMC as substrate preincubated for 30 mins followed by substrate addition by fluorometric assay | J Med Chem 59: 9457-9472 (2016) Article DOI: 10.1021/acs.jmedchem.6b01127 BindingDB Entry DOI: 10.7270/Q2Z321K4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase I (Homo sapiens (Human)) | BDBM50195244 (CHEMBL3926554 | US10287258, Example 24 | US1066924...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 13.8 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description The activity of DPP1 was determined by measuring the enzymatic release of aminomethyl coumarin (AMC) from the peptide substrate (H-Gly-Arg-AMC), whic... | US Patent US10669245 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase I (Homo sapiens (Human)) | BDBM50195244 (CHEMBL3926554 | US10287258, Example 24 | US1066924...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 13.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The activity of DPP1 was determined by measuring the enzymatic release of aminomethyl coumarin (AMC) from the peptide substrate (H-Gly-Arg-AMC), whic... | J Med Chem 51: 7049-52 (2008) BindingDB Entry DOI: 10.7270/Q2RJ4MTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase I (Homo sapiens (Human)) | BDBM50195244 (CHEMBL3926554 | US10287258, Example 24 | US1066924...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
CHARLES RIVER DISCOVERY RESEARCH SERVICES UK LIMITED Curated by ChEMBL | Assay Description Inhibition of DPP1 in human U937 cells using Gly-Phe-AFC as substrate preincubated for 60 mins followed by substrate addition by fluorescence assay | J Med Chem 59: 9457-9472 (2016) Article DOI: 10.1021/acs.jmedchem.6b01127 BindingDB Entry DOI: 10.7270/Q2Z321K4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||