 Found 18 hits for monomerid = 50199463
Found 18 hits for monomerid = 50199463 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50199463
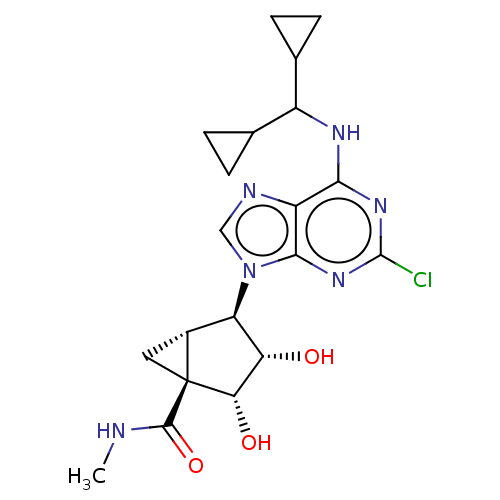
(CHEMBL3976121)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC(C3CC3)C3CC3)nc(Cl)nc12)C(=O)NC |r| Show InChI InChI=1S/C20H25ClN6O3/c1-22-18(30)20-6-10(20)13(14(28)15(20)29)27-7-23-12-16(25-19(21)26-17(12)27)24-11(8-2-3-8)9-4-5-9/h7-11,13-15,28-29H,2-6H2,1H3,(H,22,30)(H,24,25,26)/t10-,13-,14+,15+,20+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT2BR expressed in Flp-In HEK cells assessed as inhibition of 5-HT-induced calcium mobilization preincubated for 5 to ... |
J Med Chem 59: 11006-11026 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01183
BindingDB Entry DOI: 10.7270/Q2DN4709 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50199463

(CHEMBL3976121)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC(C3CC3)C3CC3)nc(Cl)nc12)C(=O)NC |r| Show InChI InChI=1S/C20H25ClN6O3/c1-22-18(30)20-6-10(20)13(14(28)15(20)29)27-7-23-12-16(25-19(21)26-17(12)27)24-11(8-2-3-8)9-4-5-9/h7-11,13-15,28-29H,2-6H2,1H3,(H,22,30)(H,24,25,26)/t10-,13-,14+,15+,20+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]lysergic from human 5-HT2BR expressed in HEK cell membranes incubated in dark for 90 mins by microbeta scintillation counting met... |
J Med Chem 59: 11006-11026 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01183
BindingDB Entry DOI: 10.7270/Q2DN4709 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50199463

(CHEMBL3976121)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC(C3CC3)C3CC3)nc(Cl)nc12)C(=O)NC |r| Show InChI InChI=1S/C20H25ClN6O3/c1-22-18(30)20-6-10(20)13(14(28)15(20)29)27-7-23-12-16(25-19(21)26-17(12)27)24-11(8-2-3-8)9-4-5-9/h7-11,13-15,28-29H,2-6H2,1H3,(H,22,30)(H,24,25,26)/t10-,13-,14+,15+,20+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Inhibition of 5HT2B (unknown origin) |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662 |
More data for this
Ligand-Target Pair | |
Transmembrane domain-containing protein TMIGD3
(Homo sapiens (Human)) | BDBM50199463

(CHEMBL3976121)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC(C3CC3)C3CC3)nc(Cl)nc12)C(=O)NC |r| Show InChI InChI=1S/C20H25ClN6O3/c1-22-18(30)20-6-10(20)13(14(28)15(20)29)27-7-23-12-16(25-19(21)26-17(12)27)24-11(8-2-3-8)9-4-5-9/h7-11,13-15,28-29H,2-6H2,1H3,(H,22,30)(H,24,25,26)/t10-,13-,14+,15+,20+/m1/s1 | UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [125l]N 6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from recombinant human A3AR expressed in CHO cell membranes after 60 m... |
J Med Chem 59: 11006-11026 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01183
BindingDB Entry DOI: 10.7270/Q2DN4709 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50199463

(CHEMBL3976121)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC(C3CC3)C3CC3)nc(Cl)nc12)C(=O)NC |r| Show InChI InChI=1S/C20H25ClN6O3/c1-22-18(30)20-6-10(20)13(14(28)15(20)29)27-7-23-12-16(25-19(21)26-17(12)27)24-11(8-2-3-8)9-4-5-9/h7-11,13-15,28-29H,2-6H2,1H3,(H,22,30)(H,24,25,26)/t10-,13-,14+,15+,20+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5-N-methyluronamide from human A3A adenosine receptor expressed in CHO cell membranes after ... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50199463

(CHEMBL3976121)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC(C3CC3)C3CC3)nc(Cl)nc12)C(=O)NC |r| Show InChI InChI=1S/C20H25ClN6O3/c1-22-18(30)20-6-10(20)13(14(28)15(20)29)27-7-23-12-16(25-19(21)26-17(12)27)24-11(8-2-3-8)9-4-5-9/h7-11,13-15,28-29H,2-6H2,1H3,(H,22,30)(H,24,25,26)/t10-,13-,14+,15+,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]N6-R-phenylisopropyladenosine from human A1A adenosine receptor expressed in CHO cell membranes after 60 mins by scintillation pr... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50199463

(CHEMBL3976121)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC(C3CC3)C3CC3)nc(Cl)nc12)C(=O)NC |r| Show InChI InChI=1S/C20H25ClN6O3/c1-22-18(30)20-6-10(20)13(14(28)15(20)29)27-7-23-12-16(25-19(21)26-17(12)27)24-11(8-2-3-8)9-4-5-9/h7-11,13-15,28-29H,2-6H2,1H3,(H,22,30)(H,24,25,26)/t10-,13-,14+,15+,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]N6-phenylisopropyladenosine from recombinant human A1AR expressed in CHO cell membranes after 60 mins by liquid scintillation cou... |
J Med Chem 59: 11006-11026 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01183
BindingDB Entry DOI: 10.7270/Q2DN4709 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50199463

(CHEMBL3976121)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC(C3CC3)C3CC3)nc(Cl)nc12)C(=O)NC |r| Show InChI InChI=1S/C20H25ClN6O3/c1-22-18(30)20-6-10(20)13(14(28)15(20)29)27-7-23-12-16(25-19(21)26-17(12)27)24-11(8-2-3-8)9-4-5-9/h7-11,13-15,28-29H,2-6H2,1H3,(H,22,30)(H,24,25,26)/t10-,13-,14+,15+,20+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 269 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human 5-HT2CR expressed in Flp-In HEK cell membranes incubated for 90 mins under dark condition by microbeta sci... |
J Med Chem 59: 11006-11026 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01183
BindingDB Entry DOI: 10.7270/Q2DN4709 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50199463

(CHEMBL3976121)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC(C3CC3)C3CC3)nc(Cl)nc12)C(=O)NC |r| Show InChI InChI=1S/C20H25ClN6O3/c1-22-18(30)20-6-10(20)13(14(28)15(20)29)27-7-23-12-16(25-19(21)26-17(12)27)24-11(8-2-3-8)9-4-5-9/h7-11,13-15,28-29H,2-6H2,1H3,(H,22,30)(H,24,25,26)/t10-,13-,14+,15+,20+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 749 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Inhibition of 5HT2C (unknown origin) |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50199463

(CHEMBL3976121)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC(C3CC3)C3CC3)nc(Cl)nc12)C(=O)NC |r| Show InChI InChI=1S/C20H25ClN6O3/c1-22-18(30)20-6-10(20)13(14(28)15(20)29)27-7-23-12-16(25-19(21)26-17(12)27)24-11(8-2-3-8)9-4-5-9/h7-11,13-15,28-29H,2-6H2,1H3,(H,22,30)(H,24,25,26)/t10-,13-,14+,15+,20+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 752 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT2CR expressed in Flp-In HEK cells assessed as inhibition of serotonin induced calcium mobilization preincubated for ... |
J Med Chem 59: 11006-11026 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01183
BindingDB Entry DOI: 10.7270/Q2DN4709 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50199463

(CHEMBL3976121)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC(C3CC3)C3CC3)nc(Cl)nc12)C(=O)NC |r| Show InChI InChI=1S/C20H25ClN6O3/c1-22-18(30)20-6-10(20)13(14(28)15(20)29)27-7-23-12-16(25-19(21)26-17(12)27)24-11(8-2-3-8)9-4-5-9/h7-11,13-15,28-29H,2-6H2,1H3,(H,22,30)(H,24,25,26)/t10-,13-,14+,15+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]2-[p-(2-carboxyethyl)phenylethylamino]-5-N-ethylcarboxamidoadenosine from human A2A adenosine receptor expressed in HEK293 cell m... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50199463

(CHEMBL3976121)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC(C3CC3)C3CC3)nc(Cl)nc12)C(=O)NC |r| Show InChI InChI=1S/C20H25ClN6O3/c1-22-18(30)20-6-10(20)13(14(28)15(20)29)27-7-23-12-16(25-19(21)26-17(12)27)24-11(8-2-3-8)9-4-5-9/h7-11,13-15,28-29H,2-6H2,1H3,(H,22,30)(H,24,25,26)/t10-,13-,14+,15+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3 H]2-[p-(2-carboxyethyl)phenyl-ethylamino]-5'-N-ethylcarboxamidoadenosine from recombinant human adenosine receptor A2A expressed i... |
J Med Chem 59: 11006-11026 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01183
BindingDB Entry DOI: 10.7270/Q2DN4709 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50199463

(CHEMBL3976121)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC(C3CC3)C3CC3)nc(Cl)nc12)C(=O)NC |r| Show InChI InChI=1S/C20H25ClN6O3/c1-22-18(30)20-6-10(20)13(14(28)15(20)29)27-7-23-12-16(25-19(21)26-17(12)27)24-11(8-2-3-8)9-4-5-9/h7-11,13-15,28-29H,2-6H2,1H3,(H,22,30)(H,24,25,26)/t10-,13-,14+,15+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5-HT2A receptor expressed in HEK-T cell membranes incubated for 90 mins under dark condition by microbeta s... |
J Med Chem 59: 11006-11026 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01183
BindingDB Entry DOI: 10.7270/Q2DN4709 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50199463

(CHEMBL3976121)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC(C3CC3)C3CC3)nc(Cl)nc12)C(=O)NC |r| Show InChI InChI=1S/C20H25ClN6O3/c1-22-18(30)20-6-10(20)13(14(28)15(20)29)27-7-23-12-16(25-19(21)26-17(12)27)24-11(8-2-3-8)9-4-5-9/h7-11,13-15,28-29H,2-6H2,1H3,(H,22,30)(H,24,25,26)/t10-,13-,14+,15+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 59: 11006-11026 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01183
BindingDB Entry DOI: 10.7270/Q2DN4709 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50199463

(CHEMBL3976121)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC(C3CC3)C3CC3)nc(Cl)nc12)C(=O)NC |r| Show InChI InChI=1S/C20H25ClN6O3/c1-22-18(30)20-6-10(20)13(14(28)15(20)29)27-7-23-12-16(25-19(21)26-17(12)27)24-11(8-2-3-8)9-4-5-9/h7-11,13-15,28-29H,2-6H2,1H3,(H,22,30)(H,24,25,26)/t10-,13-,14+,15+,20+/m1/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 59: 11006-11026 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01183
BindingDB Entry DOI: 10.7270/Q2DN4709 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50199463

(CHEMBL3976121)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC(C3CC3)C3CC3)nc(Cl)nc12)C(=O)NC |r| Show InChI InChI=1S/C20H25ClN6O3/c1-22-18(30)20-6-10(20)13(14(28)15(20)29)27-7-23-12-16(25-19(21)26-17(12)27)24-11(8-2-3-8)9-4-5-9/h7-11,13-15,28-29H,2-6H2,1H3,(H,22,30)(H,24,25,26)/t10-,13-,14+,15+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 59: 11006-11026 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01183
BindingDB Entry DOI: 10.7270/Q2DN4709 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A
(Homo sapiens (Human)) | BDBM50199463

(CHEMBL3976121)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC(C3CC3)C3CC3)nc(Cl)nc12)C(=O)NC |r| Show InChI InChI=1S/C20H25ClN6O3/c1-22-18(30)20-6-10(20)13(14(28)15(20)29)27-7-23-12-16(25-19(21)26-17(12)27)24-11(8-2-3-8)9-4-5-9/h7-11,13-15,28-29H,2-6H2,1H3,(H,22,30)(H,24,25,26)/t10-,13-,14+,15+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 59: 11006-11026 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01183
BindingDB Entry DOI: 10.7270/Q2DN4709 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50199463

(CHEMBL3976121)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC(C3CC3)C3CC3)nc(Cl)nc12)C(=O)NC |r| Show InChI InChI=1S/C20H25ClN6O3/c1-22-18(30)20-6-10(20)13(14(28)15(20)29)27-7-23-12-16(25-19(21)26-17(12)27)24-11(8-2-3-8)9-4-5-9/h7-11,13-15,28-29H,2-6H2,1H3,(H,22,30)(H,24,25,26)/t10-,13-,14+,15+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 59: 11006-11026 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01183
BindingDB Entry DOI: 10.7270/Q2DN4709 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data