 Found 6 hits for monomerid = 50199518
Found 6 hits for monomerid = 50199518 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50199518
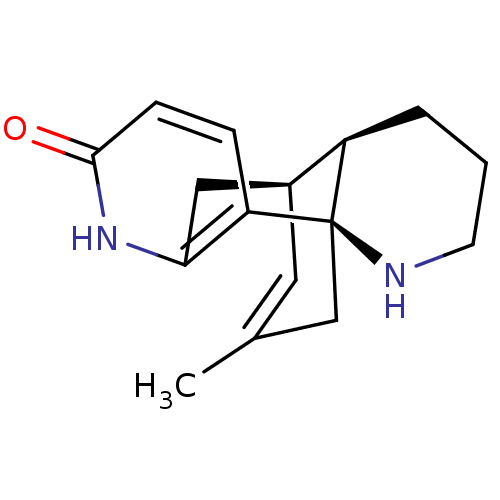
((-)-huperzine B | CHEMBL245079 | huperzine B)Show SMILES CC1=C[C@H]2Cc3[nH]c(=O)ccc3[C@]3(C1)NCCC[C@H]23 |r,t:1,THB:0:1:4.5.11:18| Show InChI InChI=1S/C16H20N2O/c1-10-7-11-8-14-13(4-5-15(19)18-14)16(9-10)12(11)3-2-6-17-16/h4-5,7,11-12,17H,2-3,6,8-9H2,1H3,(H,18,19)/t11-,12+,16+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 334 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Torpedo californica AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50199518

((-)-huperzine B | CHEMBL245079 | huperzine B)Show SMILES CC1=C[C@H]2Cc3[nH]c(=O)ccc3[C@]3(C1)NCCC[C@H]23 |r,t:1,THB:0:1:4.5.11:18| Show InChI InChI=1S/C16H20N2O/c1-10-7-11-8-14-13(4-5-15(19)18-14)16(9-10)12(11)3-2-6-17-16/h4-5,7,11-12,17H,2-3,6,8-9H2,1H3,(H,18,19)/t11-,12+,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory constant against fetal bovine serum (FBS) acetylcholinesterase |
Bioorg Med Chem Lett 12: 1521-3 (2002)
BindingDB Entry DOI: 10.7270/Q2FX79ZW |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50199518

((-)-huperzine B | CHEMBL245079 | huperzine B)Show SMILES CC1=C[C@H]2Cc3[nH]c(=O)ccc3[C@]3(C1)NCCC[C@H]23 |r,t:1,THB:0:1:4.5.11:18| Show InChI InChI=1S/C16H20N2O/c1-10-7-11-8-14-13(4-5-15(19)18-14)16(9-10)12(11)3-2-6-17-16/h4-5,7,11-12,17H,2-3,6,8-9H2,1H3,(H,18,19)/t11-,12+,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant AChE. |
Bioorg Med Chem Lett 12: 2565-8 (2002)
BindingDB Entry DOI: 10.7270/Q2CZ37Q4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50199518

((-)-huperzine B | CHEMBL245079 | huperzine B)Show SMILES CC1=C[C@H]2Cc3[nH]c(=O)ccc3[C@]3(C1)NCCC[C@H]23 |r,t:1,THB:0:1:4.5.11:18| Show InChI InChI=1S/C16H20N2O/c1-10-7-11-8-14-13(4-5-15(19)18-14)16(9-10)12(11)3-2-6-17-16/h4-5,7,11-12,17H,2-3,6,8-9H2,1H3,(H,18,19)/t11-,12+,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced neuromuscular block in guinea pig hemi-diaphragm. |
Bioorg Med Chem Lett 12: 2565-8 (2002)
BindingDB Entry DOI: 10.7270/Q2CZ37Q4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50199518

((-)-huperzine B | CHEMBL245079 | huperzine B)Show SMILES CC1=C[C@H]2Cc3[nH]c(=O)ccc3[C@]3(C1)NCCC[C@H]23 |r,t:1,THB:0:1:4.5.11:18| Show InChI InChI=1S/C16H20N2O/c1-10-7-11-8-14-13(4-5-15(19)18-14)16(9-10)12(11)3-2-6-17-16/h4-5,7,11-12,17H,2-3,6,8-9H2,1H3,(H,18,19)/t11-,12+,16+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.93E+4 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of AChE in rat cortex at pH 5 |
Bioorg Med Chem 15: 1394-408 (2007)
Article DOI: 10.1016/j.bmc.2006.11.009
BindingDB Entry DOI: 10.7270/Q27W6BTX |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Rattus norvegicus (rat)) | BDBM50199518

((-)-huperzine B | CHEMBL245079 | huperzine B)Show SMILES CC1=C[C@H]2Cc3[nH]c(=O)ccc3[C@]3(C1)NCCC[C@H]23 |r,t:1,THB:0:1:4.5.11:18| Show InChI InChI=1S/C16H20N2O/c1-10-7-11-8-14-13(4-5-15(19)18-14)16(9-10)12(11)3-2-6-17-16/h4-5,7,11-12,17H,2-3,6,8-9H2,1H3,(H,18,19)/t11-,12+,16+/m0/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in rat serum |
Bioorg Med Chem 15: 1394-408 (2007)
Article DOI: 10.1016/j.bmc.2006.11.009
BindingDB Entry DOI: 10.7270/Q27W6BTX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data