

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50199800 CHEMBL3979590::US10913711, Compound 9g::US9862679, Compound 9g
SMILES: OC(CN1CCN(CCSC(c2ccccc2)c2ccccc2)CC1)Cc1ccccc1
InChI Key: InChIKey=JGNNQJNURHXTCG-UHFFFAOYSA-N
Data: 12 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50199800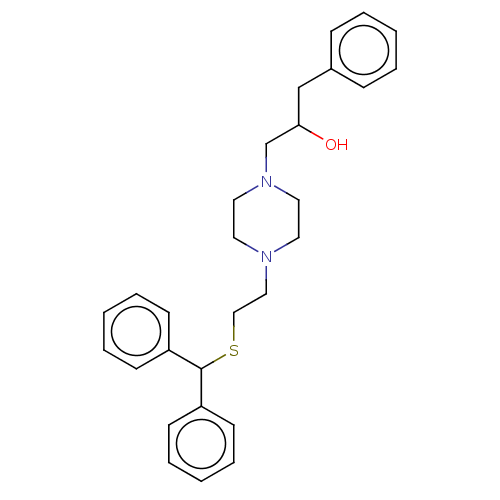 (CHEMBL3979590 | US10913711, Compound 9g | US986267...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [3H]WIN35,428 from DAT in Sprague-Dawley rat brain membranes incubated for 120 mins by radioligand binding assay | J Med Chem 59: 10676-10691 (2016) Article DOI: 10.1021/acs.jmedchem.6b01373 BindingDB Entry DOI: 10.7270/Q2WQ05RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50199800 (CHEMBL3979590 | US10913711, Compound 9g | US986267...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 2.54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE UNITED STATES OF AMERICA, AS REPRESENTED BY THE SECRETARY, DEPARTMENT OF HEALTH AND HUMAN SERVICES US Patent | Assay Description Dopamine Transporter Binding Assay. Brains from male Sprague-Dawley rats weighing 200-225 g (Taconic Labs) were removed, striatum dissected and quick... | US Patent US10913711 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50199800 (CHEMBL3979590 | US10913711, Compound 9g | US986267...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 2.54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description Brains from male Sprague-Dawley rats weighing 200-225 g (Taconic Labs) were removed, striatum dissected and quickly frozen. Membranes were prepared b... | J Med Chem 48: 7688-707 (2005) BindingDB Entry DOI: 10.7270/Q2PR7Z87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50199800 (CHEMBL3979590 | US10913711, Compound 9g | US986267...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The uptake assays were carried out 2 days after transfection. Prior to the experiment, the cells were washed once in 500 ul of uptake buffer (25 mM 4... | J Med Chem 48: 7688-707 (2005) BindingDB Entry DOI: 10.7270/Q2PR7Z87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50199800 (CHEMBL3979590 | US10913711, Compound 9g | US986267...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE UNITED STATES OF AMERICA, AS REPRESENTED BY THE SECRETARY, DEPARTMENT OF HEALTH AND HUMAN SERVICES US Patent | US Patent US10913711 (2021) | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Rattus norvegicus (Rat)) | BDBM50199800 (CHEMBL3979590 | US10913711, Compound 9g | US986267...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 31.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE UNITED STATES OF AMERICA, AS REPRESENTED BY THE SECRETARY, DEPARTMENT OF HEALTH AND HUMAN SERVICES US Patent | US Patent US10913711 (2021) | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma-2 receptor (Rattus norvegicus (Rat)) | BDBM50199800 (CHEMBL3979590 | US10913711, Compound 9g | US986267...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 31.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The uptake assays were carried out 2 days after transfection. Prior to the experiment, the cells were washed once in 500 ul of uptake buffer (25 mM 4... | J Med Chem 48: 7688-707 (2005) BindingDB Entry DOI: 10.7270/Q2PR7Z87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuroepithelial cell-transforming 1 (Rat) | BDBM50199800 (CHEMBL3979590 | US10913711, Compound 9g | US986267...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE UNITED STATES OF AMERICA, AS REPRESENTED BY THE SECRETARY, DEPARTMENT OF HEALTH AND HUMAN SERVICES US Patent | Assay Description Norepinephrine Transporter Binding Assay. Brains from male Sprague-Dawley rats weighing 200-225 g (Taconic Labs, Germantown, N.Y.) were removed, fron... | US Patent US10913711 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus (rat)) | BDBM50199800 (CHEMBL3979590 | US10913711, Compound 9g | US986267...) | Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description Brains from male Sprague-Dawley rats weighing 200-225 g (Taconic Labs, Germantown, N.Y.) were removed, frontal cortex dissected and rapidly frozen. M... | J Med Chem 48: 7688-707 (2005) BindingDB Entry DOI: 10.7270/Q2PR7Z87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50199800 (CHEMBL3979590 | US10913711, Compound 9g | US986267...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in Sprague-Dawley rat brain stem membranes incubated for 60 mins by radioligand binding assay | J Med Chem 59: 10676-10691 (2016) Article DOI: 10.1021/acs.jmedchem.6b01373 BindingDB Entry DOI: 10.7270/Q2WQ05RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50199800 (CHEMBL3979590 | US10913711, Compound 9g | US986267...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE UNITED STATES OF AMERICA, AS REPRESENTED BY THE SECRETARY, DEPARTMENT OF HEALTH AND HUMAN SERVICES US Patent | Assay Description Serotonin Transporter Binding Assay. Brains from male Sprague-Dawley rats weighing 200-225 g (Taconic Labs, Germantown, N.Y.) were removed, midbrain ... | US Patent US10913711 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50199800 (CHEMBL3979590 | US10913711, Compound 9g | US986267...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description Brains from male Sprague-Dawley rats weighing 200-225 g (Taconic Labs, Germantown, N.Y.) were removed, midbrain dissected and rapidly frozen. Membran... | J Med Chem 48: 7688-707 (2005) BindingDB Entry DOI: 10.7270/Q2PR7Z87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||