

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50199937 2-(((7R,8R,9alphaR)-8-(3-hydroxyphenyl)-7,8-dimethyl-hexahydro-1H-pyrido[1,2-R]pyrazin-2(6H)-yl)methyl)phenol::CHEMBL218242
SMILES: C[C@H]1CN2CCN(Cc3ccccc3O)C[C@H]2C[C@@]1(C)c1cccc(O)c1
InChI Key: InChIKey=VOSOSNSMMSOIES-XTQVGHSUSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50199937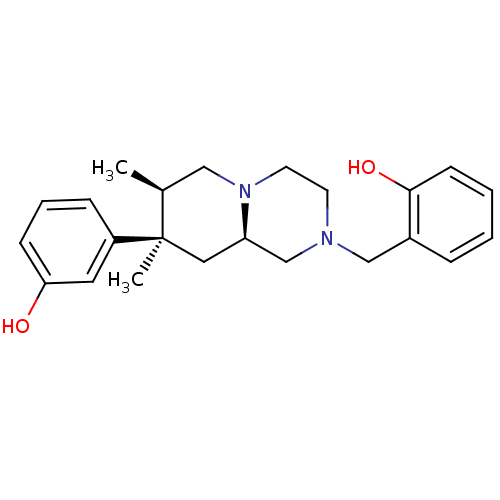 (2-(((7R,8R,9alphaR)-8-(3-hydroxyphenyl)-7,8-dimeth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human cloned mu opioid receptor expressed in CHO cells | J Med Chem 49: 7290-306 (2006) Article DOI: 10.1021/jm0604878 BindingDB Entry DOI: 10.7270/Q2CF9QW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50199937 (2-(((7R,8R,9alphaR)-8-(3-hydroxyphenyl)-7,8-dimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Antagonist activity assessed as inhibition of U50488-stimulated [35S]GTP-gamma-S binding to human kappa opioid receptor expressed in CHO cells | J Med Chem 49: 7290-306 (2006) Article DOI: 10.1021/jm0604878 BindingDB Entry DOI: 10.7270/Q2CF9QW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50199937 (2-(((7R,8R,9alphaR)-8-(3-hydroxyphenyl)-7,8-dimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human cloned delta opioid receptor expressed in CHO cells | J Med Chem 49: 7290-306 (2006) Article DOI: 10.1021/jm0604878 BindingDB Entry DOI: 10.7270/Q2CF9QW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50199937 (2-(((7R,8R,9alphaR)-8-(3-hydroxyphenyl)-7,8-dimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Antagonist activity assessed as inhibition of U50488-stimulated [35S]GTP-gamma-S binding to human kappa opioid receptor expressed in CHO cells | J Med Chem 49: 7290-306 (2006) Article DOI: 10.1021/jm0604878 BindingDB Entry DOI: 10.7270/Q2CF9QW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50199937 (2-(((7R,8R,9alphaR)-8-(3-hydroxyphenyl)-7,8-dimeth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Antagonist activity assessed as inhibition of loperamide-stimulated [35S]GTPgammaS binding to human mu opioid receptor expressed in CHO cells | J Med Chem 49: 7290-306 (2006) Article DOI: 10.1021/jm0604878 BindingDB Entry DOI: 10.7270/Q2CF9QW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50199937 (2-(((7R,8R,9alphaR)-8-(3-hydroxyphenyl)-7,8-dimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Antagonist activity assessed as inhibition of BW373U86-stimulated [35S]GTP-gamma-S binding to human delta opioid receptor expressed in CHO cells | J Med Chem 49: 7290-306 (2006) Article DOI: 10.1021/jm0604878 BindingDB Entry DOI: 10.7270/Q2CF9QW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||