

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50202115 CHEMBL217957::H-Tyr-D-Ala-Gly-Phe-NH-NH-D-Trp-Nle-Asp-Phe-Bo
SMILES: CCCC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)N[C@H](Cc1cc2ccccc2[nH]1)C(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1
InChI Key: InChIKey=FEOAQCGOOODCEB-RXFVQNAMSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50202115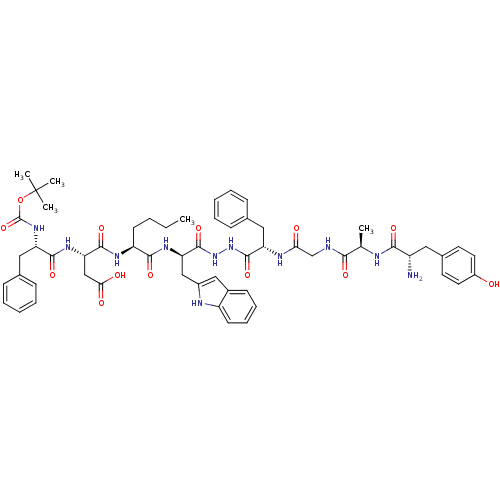 (CHEMBL217957 | H-Tyr-D-Ala-Gly-Phe-NH-NH-D-Trp-Nle...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human delta opioid receptor expressed in HN9.10 cells | J Med Chem 50: 165-8 (2007) Article DOI: 10.1021/jm061268p BindingDB Entry DOI: 10.7270/Q2TD9X17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50202115 (CHEMBL217957 | H-Tyr-D-Ala-Gly-Phe-NH-NH-D-Trp-Nle...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Activity at rat mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | J Med Chem 50: 165-8 (2007) Article DOI: 10.1021/jm061268p BindingDB Entry DOI: 10.7270/Q2TD9X17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50202115 (CHEMBL217957 | H-Tyr-D-Ala-Gly-Phe-NH-NH-D-Trp-Nle...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in HN9.10 cells | J Med Chem 50: 165-8 (2007) Article DOI: 10.1021/jm061268p BindingDB Entry DOI: 10.7270/Q2TD9X17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor (Homo sapiens (Human)) | BDBM50202115 (CHEMBL217957 | H-Tyr-D-Ala-Gly-Phe-NH-NH-D-Trp-Nle...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I]CCK8-SO3 from human CCK2 receptor expressed in HEK293 cells | J Med Chem 50: 165-8 (2007) Article DOI: 10.1021/jm061268p BindingDB Entry DOI: 10.7270/Q2TD9X17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor (Homo sapiens (Human)) | BDBM50202115 (CHEMBL217957 | H-Tyr-D-Ala-Gly-Phe-NH-NH-D-Trp-Nle...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I]CCK8-SO3 from human CCK2 receptor expressed in HEK293 cells | J Med Chem 50: 165-8 (2007) Article DOI: 10.1021/jm061268p BindingDB Entry DOI: 10.7270/Q2TD9X17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor (Homo sapiens (Human)) | BDBM50202115 (CHEMBL217957 | H-Tyr-D-Ala-Gly-Phe-NH-NH-D-Trp-Nle...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I]CCK8-SO3 from human CCK1 receptor expressed in HEK293 cells | J Med Chem 50: 165-8 (2007) Article DOI: 10.1021/jm061268p BindingDB Entry DOI: 10.7270/Q2TD9X17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor (Homo sapiens (Human)) | BDBM50202115 (CHEMBL217957 | H-Tyr-D-Ala-Gly-Phe-NH-NH-D-Trp-Nle...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I]CCK8-SO3 from human CCK1 receptor expressed in HEK293 cells | J Med Chem 50: 165-8 (2007) Article DOI: 10.1021/jm061268p BindingDB Entry DOI: 10.7270/Q2TD9X17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50202115 (CHEMBL217957 | H-Tyr-D-Ala-Gly-Phe-NH-NH-D-Trp-Nle...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Activity at delta opioid receptor in ICR mouse by MVD assay | J Med Chem 50: 165-8 (2007) Article DOI: 10.1021/jm061268p BindingDB Entry DOI: 10.7270/Q2TD9X17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50202115 (CHEMBL217957 | H-Tyr-D-Ala-Gly-Phe-NH-NH-D-Trp-Nle...) | UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Activity at mu opioid receptor assessed as contraction of electrically-stimulated Hartley guinea pig ileum | J Med Chem 50: 165-8 (2007) Article DOI: 10.1021/jm061268p BindingDB Entry DOI: 10.7270/Q2TD9X17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50202115 (CHEMBL217957 | H-Tyr-D-Ala-Gly-Phe-NH-NH-D-Trp-Nle...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Activity at human delta opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | J Med Chem 50: 165-8 (2007) Article DOI: 10.1021/jm061268p BindingDB Entry DOI: 10.7270/Q2TD9X17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50202115 (CHEMBL217957 | H-Tyr-D-Ala-Gly-Phe-NH-NH-D-Trp-Nle...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 630 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Activity at rat mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | J Med Chem 50: 165-8 (2007) Article DOI: 10.1021/jm061268p BindingDB Entry DOI: 10.7270/Q2TD9X17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||