

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50202367 CHEMBL374108::cyclo(-D-Tyr-L-Ala-L-Arg-L-Nal-Gly-)
SMILES: [#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc3ccccc3c2)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O
InChI Key: InChIKey=KSQLRHSLMODJAS-OQVMAISDSA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 4 (CXCR4) (Homo sapiens (Human)) | BDBM50202367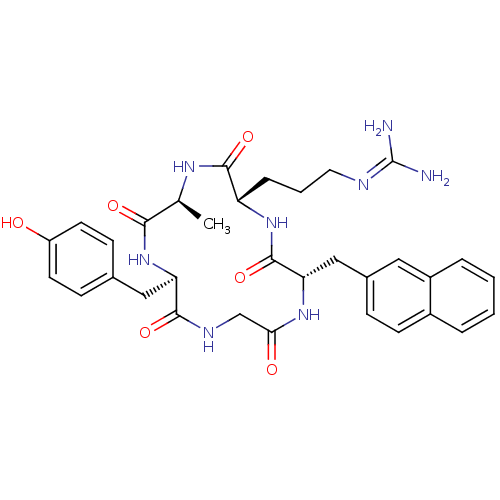 (CHEMBL374108 | cyclo(-D-Tyr-L-Ala-L-Arg-L-Nal-Gly-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]SDF1 binding to CXCR4 transfected in CHO cells | J Med Chem 50: 192-8 (2007) Article DOI: 10.1021/jm0607350 BindingDB Entry DOI: 10.7270/Q25M65D3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||