

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50203012 1-(2-bromophenyl)-3-(2-hydroxy-4-nitrophenyl)urea::CHEMBL239767::SB-225002
SMILES: Oc1cc(ccc1NC(=O)Nc1ccccc1Br)[N+]([O-])=O
InChI Key: InChIKey=MQBZVUNNWUIPMK-UHFFFAOYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50203012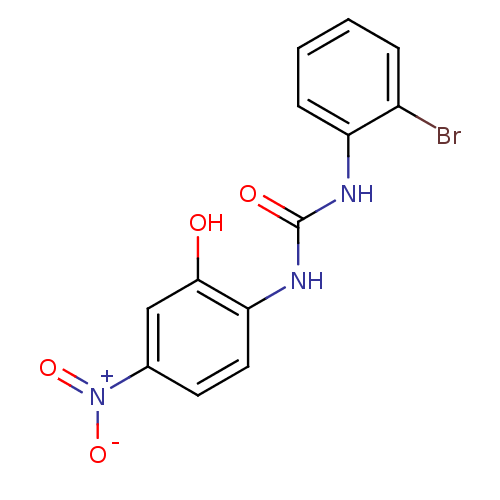 (1-(2-bromophenyl)-3-(2-hydroxy-4-nitrophenyl)urea ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntrix Biosystems Curated by ChEMBL | Assay Description Antagonist activity at human CXCR2 expressed in HEK293 cells assessed as inhibition of CXCL8-induced intracellular Ca2+ release by fluorescence based... | J Med Chem 57: 8378-97 (2014) Article DOI: 10.1021/jm500827t BindingDB Entry DOI: 10.7270/Q2FX7C22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50203012 (1-(2-bromophenyl)-3-(2-hydroxy-4-nitrophenyl)urea ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntrix Biosystems Curated by ChEMBL | Assay Description Antagonist activity at CXCR2 in human PMNs assessed as inhibition of CXCL1-induced intracellular Ca2+ release by fluorescence based calcium flux assa... | J Med Chem 57: 8378-97 (2014) Article DOI: 10.1021/jm500827t BindingDB Entry DOI: 10.7270/Q2FX7C22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50203012 (1-(2-bromophenyl)-3-(2-hydroxy-4-nitrophenyl)urea ...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute Curated by ChEMBL | Assay Description Antagonist activity at CXCR1 assessed as inhibition of CXCL8 binding by cell based assay | J Med Chem 55: 9363-92 (2012) Article DOI: 10.1021/jm300682j BindingDB Entry DOI: 10.7270/Q2862HKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50203012 (1-(2-bromophenyl)-3-(2-hydroxy-4-nitrophenyl)urea ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute Curated by ChEMBL | Assay Description Binding affinity to CXCR2 | J Med Chem 55: 9363-92 (2012) Article DOI: 10.1021/jm300682j BindingDB Entry DOI: 10.7270/Q2862HKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50203012 (1-(2-bromophenyl)-3-(2-hydroxy-4-nitrophenyl)urea ...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute Curated by ChEMBL | Assay Description Binding affinity to CXCR1 | J Med Chem 55: 9363-92 (2012) Article DOI: 10.1021/jm300682j BindingDB Entry DOI: 10.7270/Q2862HKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50203012 (1-(2-bromophenyl)-3-(2-hydroxy-4-nitrophenyl)urea ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from human recombinant CXCR2 expressed in CHO cells | Bioorg Med Chem Lett 17: 1713-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.067 BindingDB Entry DOI: 10.7270/Q2Z320GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||