

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50203122 5,8,13,13a-tetrahydro-9,10-dimethoxy-6H-benzo(g)-1,3-benzodioxolo(5,6-a)quinolizine::5,8,13,13a-tetrahydro-9,10-dimethoxy-6H-benzo(g)benzo-1,3-dioxolo(5,6-a)quinolizine::9,10-dimethoxy-2,3-(methylenedioxy)berbine::9,10-dimethoxy-5,8,13,13a-tetrahydro-6H-[1,3]dioxolo[4,5-g]isoquino[3,2-a]isoquinoline::CHEMBL275097::Canadin::canadine::tetrahydroberberine::xanthopuccine
SMILES: COc1ccc2CC3N(CCc4cc5OCOc5cc34)Cc2c1OC
InChI Key: InChIKey=VZTUIEROBZXUFA-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50203122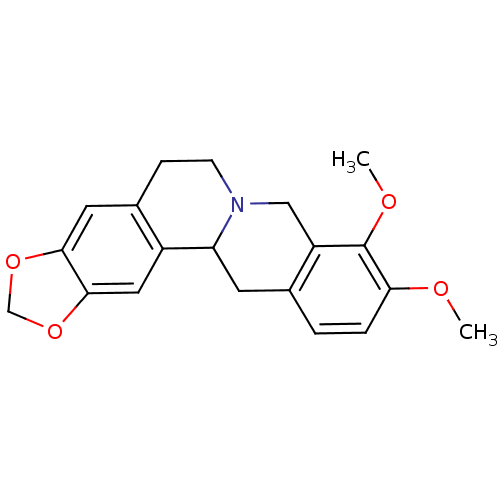 (5,8,13,13a-tetrahydro-9,10-dimethoxy-6H-benzo(g)-1...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [125I]-BE2254 from Wistar rat alpha1A adrenoceptor after 20 mins | J Med Chem 59: 9489-9502 (2016) Article DOI: 10.1021/acs.jmedchem.6b01217 BindingDB Entry DOI: 10.7270/Q2TB18VD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50203122 (5,8,13,13a-tetrahydro-9,10-dimethoxy-6H-benzo(g)-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of CDK2 | Bioorg Med Chem Lett 20: 1384-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.007 BindingDB Entry DOI: 10.7270/Q2QF8TTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50203122 (5,8,13,13a-tetrahydro-9,10-dimethoxy-6H-benzo(g)-1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.89E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Inhibition of HIV1 RT | J Nat Prod 54: 143-54 Article DOI: 10.1021/np50073a012 BindingDB Entry DOI: 10.7270/Q2NK3HTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/tissue factor (Homo sapiens (Human)) | BDBM50203122 (5,8,13,13a-tetrahydro-9,10-dimethoxy-6H-benzo(g)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of tissue factor procoagulant activity in LPS-stimulated human THP1 cells preincubated for 1 hr before LPS addition measured after 5 hrs | Bioorg Med Chem 21: 62-9 (2012) Article DOI: 10.1016/j.bmc.2012.11.002 BindingDB Entry DOI: 10.7270/Q2M32X22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||