

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50206963 CHEMBL3948337::US9518055, Example 41
SMILES: Fc1ccc2nc(c(C3CC3)n2c1)-c1cncc2ccccc12
InChI Key: InChIKey=VSSMWFPLHKIWCP-UHFFFAOYSA-N
Data: 7 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50206963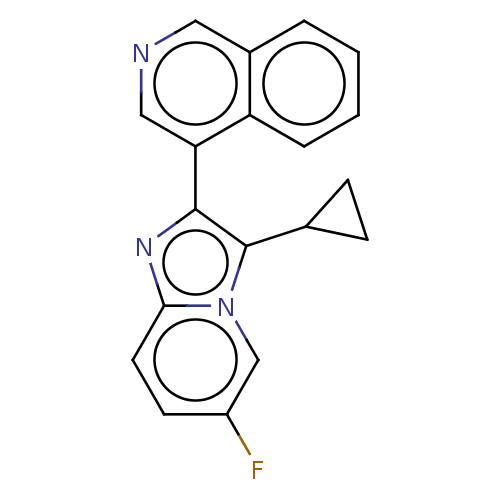 (CHEMBL3948337 | US9518055, Example 41) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck USA Curated by ChEMBL | Assay Description Inhibition of human CYP11B1-8C7 expressed in Chinese hamster V79 cells using 11-deoxycortisol as substrate preincubated for 1 hr followed by substrat... | Bioorg Med Chem Lett 27: 143-146 (2017) Article DOI: 10.1016/j.bmcl.2016.12.003 BindingDB Entry DOI: 10.7270/Q20K2BK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 19A1 (Homo sapiens (Human)) | BDBM50206963 (CHEMBL3948337 | US9518055, Example 41) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck USA Curated by ChEMBL | Assay Description Inhibition of CYP19 (unknown origin) using MFC as substrate and NADPH as cofactor preincubated for 10 mins with cofactor followed by substrate/enzyme... | Bioorg Med Chem Lett 27: 143-146 (2017) Article DOI: 10.1016/j.bmcl.2016.12.003 BindingDB Entry DOI: 10.7270/Q20K2BK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50206963 (CHEMBL3948337 | US9518055, Example 41) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck USA Curated by ChEMBL | Assay Description Inhibition of hepatic CYP3A4 (unknown origin) | Bioorg Med Chem Lett 27: 143-146 (2017) Article DOI: 10.1016/j.bmcl.2016.12.003 BindingDB Entry DOI: 10.7270/Q20K2BK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50206963 (CHEMBL3948337 | US9518055, Example 41) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 57.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme US Patent | Assay Description V79 cell lines stably expressing the either the human CYP11B2 or the human CYP11B1 enzyme were generated using a standard transfection protocol. V79 ... | US Patent US9518055 (2016) BindingDB Entry DOI: 10.7270/Q23B625B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2 (CYP11B2) (Homo sapiens (Human)) | BDBM50206963 (CHEMBL3948337 | US9518055, Example 41) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck USA Curated by ChEMBL | Assay Description Inhibition of human CYP11B2-CLE9 expressed in Chinese hamster V79 cells using 11-deoxycorticosterone as substrate preincubated for 1 hr followed by s... | Bioorg Med Chem Lett 27: 143-146 (2017) Article DOI: 10.1016/j.bmcl.2016.12.003 BindingDB Entry DOI: 10.7270/Q20K2BK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2 (CYP11B2) (Homo sapiens (Human)) | BDBM50206963 (CHEMBL3948337 | US9518055, Example 41) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme US Patent | Assay Description V79 cell lines stably expressing the either the human CYP11B2 or the human CYP11B1 enzyme were generated using a standard transfection protocol. V79 ... | US Patent US9518055 (2016) BindingDB Entry DOI: 10.7270/Q23B625B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 21 (Homo sapiens (Human)) | BDBM50206963 (CHEMBL3948337 | US9518055, Example 41) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck USA Curated by ChEMBL | Assay Description Inhibition of human CYP21 expressed in African green monkey COS7 cells using 17-hydroxypregnenolone as substrate preincubated for 1 hr followed by su... | Bioorg Med Chem Lett 27: 143-146 (2017) Article DOI: 10.1016/j.bmcl.2016.12.003 BindingDB Entry DOI: 10.7270/Q20K2BK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||