

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50211197 CHEMBL3951064
SMILES: [H][C@@]12CCN(C1)C[C@@]21CN=C(Nc2nc3ccccc3s2)O1
InChI Key: InChIKey=OZMMPPFQKQDOQW-BMIGLBTASA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor (Rattus norvegicus (Rat)) | BDBM50211197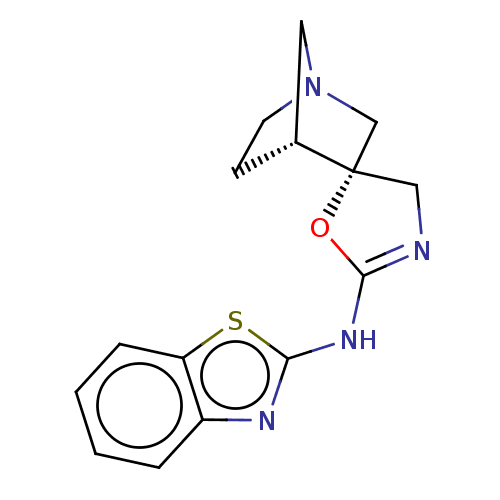 (CHEMBL3951064) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at rat alpha7 nAChR expressed in HEK293 cells co-expressing human RIC3 measured for 2 mins by FLIPR assay | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor (Rattus norvegicus (Rat)) | BDBM50211197 (CHEMBL3951064) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 280 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Partial agonist activity at rat alpha7 nAChR expressed in HEK293 cells co-expressing human RIC3 at holding potential of -90 mV by whole-cell voltage ... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50211197 (CHEMBL3951064) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT3A expressed in HEK293 cells assessed as reduction in acetylcholine-induced activity preincubated for 30 mins follow... | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||