

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50212293 (1S,9R,10R)-5-amino-20-(cyclopropylmethyl)-6-thia-4,20-diazapentacyclo[8.7.3.0^{1,9}.0^{3,7}.0^{12,17}]icosa-3(7),4,12(17),13,15-pentaen-15-ol::CHEMBL226217
SMILES: Nc1nc2C[C@]34CCN(CC5CC5)[C@H](Cc5ccc(O)cc35)[C@@H]4Cc2s1
InChI Key: InChIKey=HBUZKCMRMAMMDZ-YRISNDGFSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50212293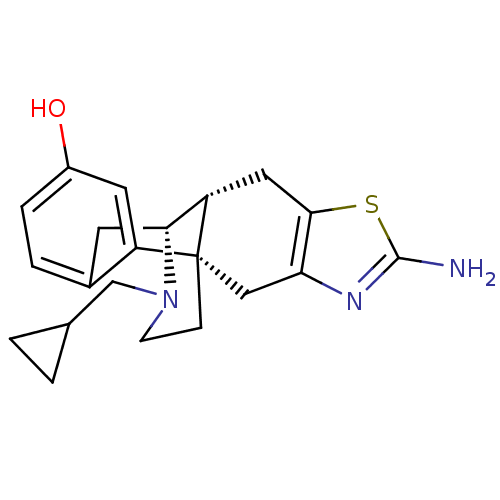 ((1S,9R,10R)-5-amino-20-(cyclopropylmethyl)-6-thia-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50212293 ((1S,9R,10R)-5-amino-20-(cyclopropylmethyl)-6-thia-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50212293 ((1S,9R,10R)-5-amino-20-(cyclopropylmethyl)-6-thia-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO membrane | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50212293 ((1S,9R,10R)-5-amino-20-(cyclopropylmethyl)-6-thia-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Agonist activity at human delta opioid receptor expressed in CHO membrane assessed as stimulation of [35S]GTP-gamma-S binding | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50212293 ((1S,9R,10R)-5-amino-20-(cyclopropylmethyl)-6-thia-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor transfected in CHO cells assessed as inhibition of U50488-induced [35S]GTPgammaS binding after 60 min... | J Med Chem 56: 8872-8 (2013) Article DOI: 10.1021/jm401290y BindingDB Entry DOI: 10.7270/Q2KH0R96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50212293 ((1S,9R,10R)-5-amino-20-(cyclopropylmethyl)-6-thia-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in CHO membrane assessed as inhibition of U50488-induced [35S]GTP-gamma-S binding | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50212293 ((1S,9R,10R)-5-amino-20-(cyclopropylmethyl)-6-thia-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO membrane assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50212293 ((1S,9R,10R)-5-amino-20-(cyclopropylmethyl)-6-thia-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor transfected in CHO cells assessed as stimulation of [35S]GTPgammaS binding after 60 mins by scintilla... | J Med Chem 56: 8872-8 (2013) Article DOI: 10.1021/jm401290y BindingDB Entry DOI: 10.7270/Q2KH0R96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50212293 ((1S,9R,10R)-5-amino-20-(cyclopropylmethyl)-6-thia-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO membrane assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | J Med Chem 50: 2747-51 (2007) Article DOI: 10.1021/jm0701674 BindingDB Entry DOI: 10.7270/Q29Z94M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||