

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50212400 1-(2,4-Dihydroxyphenyl)-3-[4-hydroxy-3-(3-methylbut-2-enyl)phenyl]-prop-2-en-1-one::1-(2,4-dihydroxyphenyl)-3-[4-hydroxy-3-(3-methylbut-2-enyl)-phenyl]-prop-2-en-1-one::CHEMBL229885::licoagrochalcone A
SMILES: [#6]\[#6](-[#6])=[#6]/[#6]-c1cc(\[#6]=[#6]\[#6](=O)-c2ccc(-[#8])cc2-[#8])ccc1-[#8]
InChI Key: InChIKey=TVUGLERLRIQATC-BJMVGYQFSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuraminidase (Influenza A virus) | BDBM50212400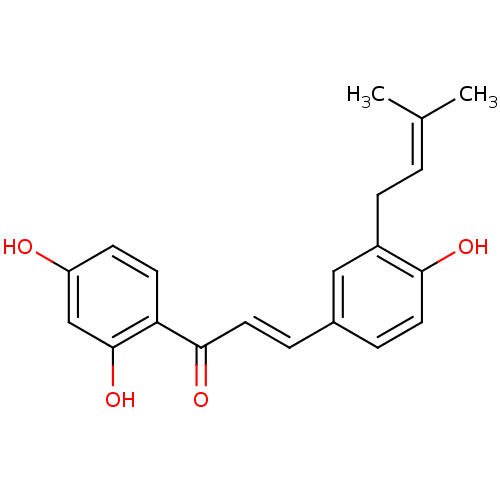 (1-(2,4-Dihydroxyphenyl)-3-[4-hydroxy-3-(3-methylbu...) | PDB MMDB B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Non-competitive inhibition of influenza A virus H1N1 Neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate sub... | Bioorg Med Chem Lett 20: 6430-4 (2010) Article DOI: 10.1016/j.bmcl.2010.09.077 BindingDB Entry DOI: 10.7270/Q2HQ42Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 19A1 (Homo sapiens (Human)) | BDBM50212400 (1-(2,4-Dihydroxyphenyl)-3-[4-hydroxy-3-(3-methylbu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human aromatase | J Med Chem 50: 2799-806 (2007) Article DOI: 10.1021/jm070109i BindingDB Entry DOI: 10.7270/Q2668F0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50212400 (1-(2,4-Dihydroxyphenyl)-3-[4-hydroxy-3-(3-methylbu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing 100191, People's Republic of China. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins | Bioorg Med Chem 25: 3706-3713 (2017) Article DOI: 10.1016/j.bmc.2017.05.009 BindingDB Entry DOI: 10.7270/Q2C82CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50212400 (1-(2,4-Dihydroxyphenyl)-3-[4-hydroxy-3-(3-methylbu...) | PDB MMDB B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of influenza A virus H1N1 Neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate substrate | Bioorg Med Chem Lett 20: 6430-4 (2010) Article DOI: 10.1016/j.bmcl.2010.09.077 BindingDB Entry DOI: 10.7270/Q2HQ42Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50212400 (1-(2,4-Dihydroxyphenyl)-3-[4-hydroxy-3-(3-methylbu...) | PDB MMDB B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase activity after 2 hrs by spectrofluorometry | Bioorg Med Chem Lett 21: 294-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.016 BindingDB Entry DOI: 10.7270/Q2N87DMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50212400 (1-(2,4-Dihydroxyphenyl)-3-[4-hydroxy-3-(3-methylbu...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Clostridium perfringens neuraminidase | Bioorg Med Chem Lett 21: 294-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.016 BindingDB Entry DOI: 10.7270/Q2N87DMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50212400 (1-(2,4-Dihydroxyphenyl)-3-[4-hydroxy-3-(3-methylbu...) | PDB MMDB B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.84E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Noncompetitive inhibition of Influenza A H1N1 virus neuraminidase activity by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 294-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.016 BindingDB Entry DOI: 10.7270/Q2N87DMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50212400 (1-(2,4-Dihydroxyphenyl)-3-[4-hydroxy-3-(3-methylbu...) | PDB MMDB B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A H9N2 virus neuraminidase activity after 2 hrs by spectrofluorometry | Bioorg Med Chem Lett 21: 294-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.016 BindingDB Entry DOI: 10.7270/Q2N87DMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50212400 (1-(2,4-Dihydroxyphenyl)-3-[4-hydroxy-3-(3-methylbu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B | J Nat Prod 70: 1039-42 (2007) Article DOI: 10.1021/np060477+ BindingDB Entry DOI: 10.7270/Q2KH0P5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50212400 (1-(2,4-Dihydroxyphenyl)-3-[4-hydroxy-3-(3-methylbu...) | PDB MMDB B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of influenza A virus H9N2 Neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate substrate | Bioorg Med Chem Lett 20: 6430-4 (2010) Article DOI: 10.1016/j.bmcl.2010.09.077 BindingDB Entry DOI: 10.7270/Q2HQ42Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||