

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50212635 CHEMBL415266
SMILES: [H][C@]12S[C@@](C)(CSc3nnnn3C)[C@@H](N1C(=O)[C@H]2Br)C(O)=O
InChI Key: InChIKey=GCUVODSDAPCLNV-ASTPYSOASA-N
Data: 10 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase (Staphylococcus aureus) | BDBM50212635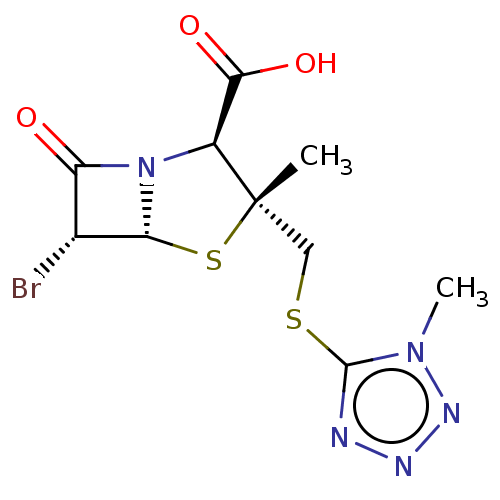 (CHEMBL415266) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Staphylococcus aureus CJ8 beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Class D β-lactamase (OXA-1) (Escherichia coli (Enterobacteria)) | BDBM50212635 (CHEMBL415266) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 8.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli HA209 Class-V OXA-1 type beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase type II (Bacteroides fragilis) | BDBM50212635 (CHEMBL415266) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 40.6 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Bacteroides fragilis 88854 Class-I beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50212635 (CHEMBL415266) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Enterobacter cloacae HC8 Class-I beta-lactamase enzyme type P99 | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (TEM-1) (Escherichia coli) | BDBM50212635 (CHEMBL415266) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli HA58R Class-III TEM-1 type beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Klebsiella oxytoca) | BDBM50212635 (CHEMBL415266) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 634 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Klebsiella oxytoca HC7 Class-IV K1 type beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Proteus mirabilis) | BDBM50212635 (CHEMBL415266) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 7.61 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Proteus vulgaris HJ33C Class-I beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Citrobacter freundii) | BDBM50212635 (CHEMBL415266) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 1.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Citrobacter freundii 87470 Class-I beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SHV-1 beta-lactamase (Klebsiella pneumoniae (Enterobacteria)) | BDBM50212635 (CHEMBL415266) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli HA208 Class-III SHV-1 type beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase BRO-1 (Moraxella catarrhalis) | BDBM50212635 (CHEMBL415266) | UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 50.7 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Branhamella catarrhalis 89001 Class-III BRO-1 type beta-lactamase enzyme | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZW1P2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||