

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50214608 CHEMBL279564::pyrene
SMILES: c1cc2ccc3cccc4ccc(c1)c2c34
InChI Key: InChIKey=BBEAQIROQSPTKN-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50214608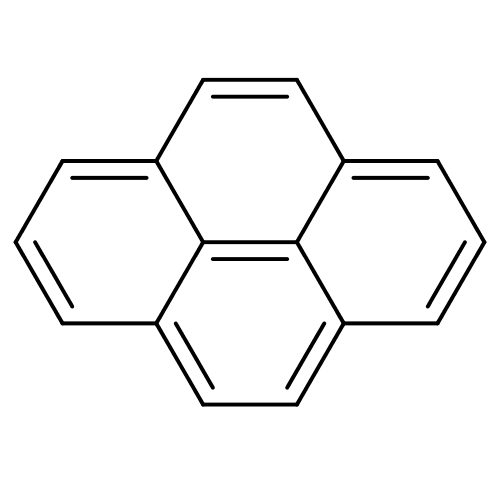 (CHEMBL279564 | pyrene) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of CYP1B1 | Bioorg Med Chem 15: 5047-60 (2007) Article DOI: 10.1016/j.bmc.2007.05.046 BindingDB Entry DOI: 10.7270/Q20R9Q7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM50214608 (CHEMBL279564 | pyrene) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of CYP1A2 | Bioorg Med Chem 15: 5047-60 (2007) Article DOI: 10.1016/j.bmc.2007.05.046 BindingDB Entry DOI: 10.7270/Q20R9Q7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polypeptide N-acetylgalactosaminyltransferase 2 (Homo sapiens (Human)) | BDBM50214608 (CHEMBL279564 | pyrene) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human recombinant FLAG-tagged ppGalNAcT2 expressed in HEK293T cells and using 5-FAM labelled-EA2 peptide as subst... | Bioorg Med Chem 27: 3372-3382 (2019) Article DOI: 10.1016/j.bmc.2019.06.020 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50214608 (CHEMBL279564 | pyrene) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of human CYP1B1 expressed in Escherichia coli DH5alpha coexpressing human NADPH P450 reductase using 7-ethoxyresorufin as substrate in pre... | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM50214608 (CHEMBL279564 | pyrene) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of human CYP1A1 expressed in Escherichia coli DH5alpha coexpressing human NADPH P450 reductase using 7-ethoxyresorufin as substrate in pre... | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50214608 (CHEMBL279564 | pyrene) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of CYP1A1 | Bioorg Med Chem 15: 5047-60 (2007) Article DOI: 10.1016/j.bmc.2007.05.046 BindingDB Entry DOI: 10.7270/Q20R9Q7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||