

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50217952 (1R,5R,9S)-(-)-9-hydroxy-5-(3-hydroxyphenyl-2-phenylethyl-2-azabicyclo[3.3.1]nonane::CHEMBL388873
SMILES: O[C@@H]1[C@H]2CCC[C@@]1(CCN2CCc1ccccc1)c1cccc(O)c1
InChI Key: InChIKey=YJDWRJHIVSZYJV-YPAWHYETSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50217952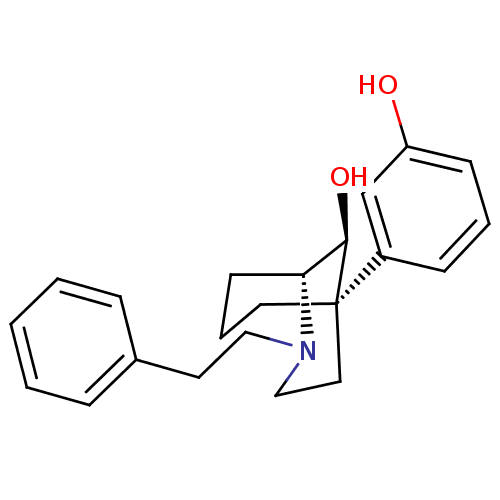 ((1R,5R,9S)-(-)-9-hydroxy-5-(3-hydroxyphenyl-2-phen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human mu opioid receptor expressed in CHO cells | J Med Chem 50: 3765-76 (2007) Article DOI: 10.1021/jm061325e BindingDB Entry DOI: 10.7270/Q2D21XB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50217952 ((1R,5R,9S)-(-)-9-hydroxy-5-(3-hydroxyphenyl-2-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.265 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Design and Synthesis Section, Molecular Targets and Medications Discovery Branch, Intramural Research Program, National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Al Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 1 hr by... | Bioorg Med Chem 25: 2406-2422 (2017) Article DOI: 10.1016/j.bmc.2017.02.064 BindingDB Entry DOI: 10.7270/Q2765HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50217952 ((1R,5R,9S)-(-)-9-hydroxy-5-(3-hydroxyphenyl-2-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human delta opioid receptor expressed in CHO cells | J Med Chem 50: 3765-76 (2007) Article DOI: 10.1021/jm061325e BindingDB Entry DOI: 10.7270/Q2D21XB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50217952 ((1R,5R,9S)-(-)-9-hydroxy-5-(3-hydroxyphenyl-2-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Design and Synthesis Section, Molecular Targets and Medications Discovery Branch, Intramural Research Program, National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Al Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from delta opioid receptor in rat brain after 60 mins by liquid scintillation counting method | Bioorg Med Chem 25: 2406-2422 (2017) Article DOI: 10.1016/j.bmc.2017.02.064 BindingDB Entry DOI: 10.7270/Q2765HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50217952 ((1R,5R,9S)-(-)-9-hydroxy-5-(3-hydroxyphenyl-2-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human kappa opioid receptor expressed in CHO cells | J Med Chem 50: 3765-76 (2007) Article DOI: 10.1021/jm061325e BindingDB Entry DOI: 10.7270/Q2D21XB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50217952 ((1R,5R,9S)-(-)-9-hydroxy-5-(3-hydroxyphenyl-2-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
Drug Design and Synthesis Section, Molecular Targets and Medications Discovery Branch, Intramural Research Program, National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Al Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 1 hr by... | Bioorg Med Chem 25: 2406-2422 (2017) Article DOI: 10.1016/j.bmc.2017.02.064 BindingDB Entry DOI: 10.7270/Q2765HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50217952 ((1R,5R,9S)-(-)-9-hydroxy-5-(3-hydroxyphenyl-2-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a |
Drug Design and Synthesis Section, Molecular Targets and Medications Discovery Branch, Intramural Research Program, National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Al Curated by ChEMBL | Assay Description Agonist activity at rat delta opioid receptor expressed in rat C6 cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 1 hr... | Bioorg Med Chem 25: 2406-2422 (2017) Article DOI: 10.1016/j.bmc.2017.02.064 BindingDB Entry DOI: 10.7270/Q2765HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||