

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50218196 3-beta,28-Dihydroxy-urs-12-ene::CHEMBL399873::Uvaol::urs-12-ene-3beta,28-diol
SMILES: C[C@@H]1CC[C@]2(CO)CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C
InChI Key: InChIKey=XUARCIYIVXVTAE-ZAPOICBTSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50218196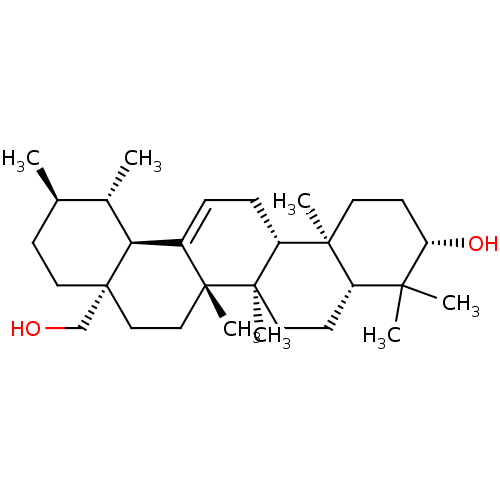 (3-beta,28-Dihydroxy-urs-12-ene | CHEMBL399873 | Uv...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B after 30 mins | J Nat Prod 71: 1775-8 (2008) Article DOI: 10.1021/np800298w BindingDB Entry DOI: 10.7270/Q2TQ62F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled bile acid receptor 1 (Homo sapiens (Human)) | BDBM50218196 (3-beta,28-Dihydroxy-urs-12-ene | CHEMBL399873 | Uv...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Universite Louis Pasteur Curated by ChEMBL | Assay Description Agonist activity at TGR5 expressed in CHO cells by CRE-driven luciferase reporter gene assay | J Med Chem 53: 178-90 (2010) Article DOI: 10.1021/jm900872z BindingDB Entry DOI: 10.7270/Q2RJ4KDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear factor NF-kappa-B (Homo sapiens (Human)) | BDBM50218196 (3-beta,28-Dihydroxy-urs-12-ene | CHEMBL399873 | Uv...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of biotinylated consensus sequence binding to NF-kB p65 in human HeLa nuclear extracts after 3 hrs by ELISA | Bioorg Med Chem 26: 4452-4460 (2018) Article DOI: 10.1016/j.bmc.2018.07.025 BindingDB Entry DOI: 10.7270/Q2N300MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50218196 (3-beta,28-Dihydroxy-urs-12-ene | CHEMBL399873 | Uv...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of TDP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) using 5'-FAM-AGGATCTAAAAGACTT-BHQ-3' as substrate preincubated for 30 mi... | Bioorg Med Chem 28: (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase/FLAP (Homo sapiens (Human)) | BDBM50218196 (3-beta,28-Dihydroxy-urs-12-ene | CHEMBL399873 | Uv...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LOX expressed in insect cells assessed as decrease in production of 5-HPETE and 5-HETE using arachidonic acid as su... | J Nat Prod 82: 3311-3320 (2019) Article DOI: 10.1021/acs.jnatprod.9b00538 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-Lipoxygenase-2 (15-LOX-2) (Homo sapiens (Human)) | BDBM50218196 (3-beta,28-Dihydroxy-urs-12-ene | CHEMBL399873 | Uv...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His-tagged 15-LOX2 expressed in Escherichia coli using arachidonic acid as substrate preincubated for 5 mi... | J Nat Prod 82: 3311-3320 (2019) Article DOI: 10.1021/acs.jnatprod.9b00538 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-Lipoxygenase-2 (15-LOX-2) (Homo sapiens (Human)) | BDBM50218196 (3-beta,28-Dihydroxy-urs-12-ene | CHEMBL399873 | Uv...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His-tagged 15-LOX2 expressed in Escherichia coli using arachidonic acid as substrate preincubated for 5 mi... | J Nat Prod 82: 3311-3320 (2019) Article DOI: 10.1021/acs.jnatprod.9b00538 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase/FLAP (Homo sapiens (Human)) | BDBM50218196 (3-beta,28-Dihydroxy-urs-12-ene | CHEMBL399873 | Uv...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LOX expressed in insect cells assessed as decrease in production of 5-HPETE and 5-HETE using arachidonic acid as su... | J Nat Prod 82: 3311-3320 (2019) Article DOI: 10.1021/acs.jnatprod.9b00538 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||