

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: O=C(NC[C@@H]1CC[C@H](CCOc2ccccc2)CC1)C1NNC(=O)C=C1
InChI Key: InChIKey=ZVCJYSAIJULKCB-BYICEURKSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50220598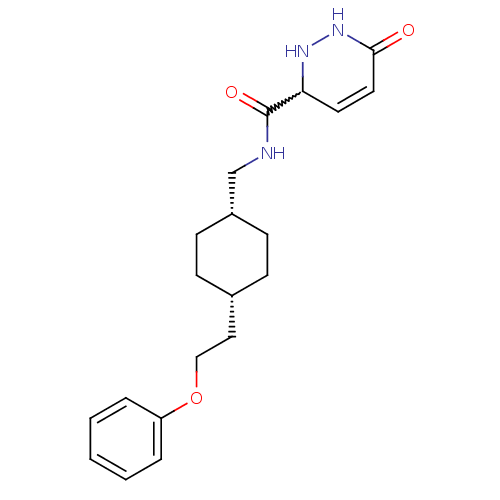 (6-oxo-N-(((1s,4s)-4-(2-phenoxyethyl)cyclohexyl)met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]racemic CP101606 from rat NR2B receptor in P2 membrane | Bioorg Med Chem Lett 17: 5537-42 (2007) Article DOI: 10.1016/j.bmcl.2007.08.033 BindingDB Entry DOI: 10.7270/Q2M908D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||