

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: COc1cc2ncnc(Nc3ccc(O)cc3)c2cc1OC
InChI Key: InChIKey=HOZUXBLMYUPGPZ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50227519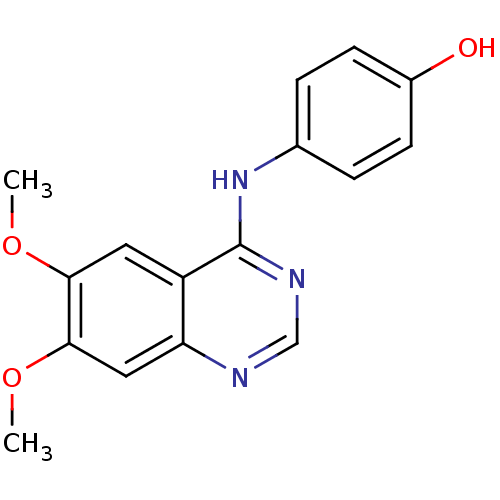 (4-(6,7-dimethoxyquinazolin-4-ylamino)phenol | CHEM...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaDesign, Inc. Curated by ChEMBL | Assay Description Inhibition of ALK using FL-Peptide 13, 5-FAM-KKSRGDYMTMQIG-CONH2 substrate after 60 mins by mobility shift assay | Bioorg Med Chem 19: 3086-95 (2011) Article DOI: 10.1016/j.bmc.2011.04.008 BindingDB Entry DOI: 10.7270/Q2JQ11B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50227519 (4-(6,7-dimethoxyquinazolin-4-ylamino)phenol | CHEM...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged human KDR expressed in insect Sf21 cells preincubated for 15 mins followed by substrate addition measured after ... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50227519 (4-(6,7-dimethoxyquinazolin-4-ylamino)phenol | CHEM...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinase suppressor of Ras 2 (Human) | BDBM50227519 (4-(6,7-dimethoxyquinazolin-4-ylamino)phenol | CHEM...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai US Patent | Assay Description The ATP-biotin assay was performed in buffer containing 25 mM Tris pH 7.5, 150 mM NaCl, 10 mM MgCl2, and 2% DMSO. Purified hKSR2-rMEK1 was assayed at... | US Patent US10548897 (2020) BindingDB Entry DOI: 10.7270/Q25H7JMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50227519 (4-(6,7-dimethoxyquinazolin-4-ylamino)phenol | CHEM...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase in Jurkat cells where p56lck autophosphorylation is inhibited. | Bioorg Med Chem Lett 7: 417-420 (1997) Article DOI: 10.1016/S0960-894X(97)00034-6 BindingDB Entry DOI: 10.7270/Q2J966VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50227519 (4-(6,7-dimethoxyquinazolin-4-ylamino)phenol | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Researchand Development Curated by ChEMBL | Assay Description Inhibition of JAK2 | Science 302: 875-878 (2003) Article DOI: 10.1126/science.1087061 BindingDB Entry DOI: 10.7270/Q22Z16FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50227519 (4-(6,7-dimethoxyquinazolin-4-ylamino)phenol | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Researchand Development Curated by ChEMBL | Assay Description Inhibition of JAK1 | Science 302: 875-878 (2003) Article DOI: 10.1126/science.1087061 BindingDB Entry DOI: 10.7270/Q22Z16FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50227519 (4-(6,7-dimethoxyquinazolin-4-ylamino)phenol | CHEM...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Epidermal growth factor receptor autophosphorylation. | Bioorg Med Chem Lett 7: 417-420 (1997) Article DOI: 10.1016/S0960-894X(97)00034-6 BindingDB Entry DOI: 10.7270/Q2J966VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||