

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50229429 CHEMBL4092663
SMILES: CN(Cc1cc(F)cc(F)c1F)C(=O)C1(CCC1)C(F)(F)F
InChI Key: InChIKey=PSPFBXRXYPDHDJ-UHFFFAOYSA-N
Data: 1 EC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50229429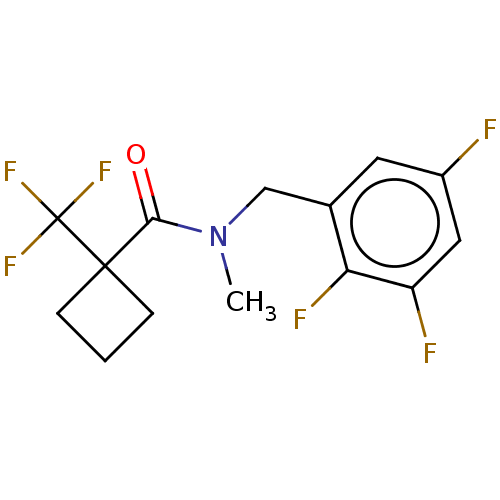 (CHEMBL4092663) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 656 | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences Curated by ChEMBL | Assay Description Inhibition of RIP1 in human HT-29 cells assessed as reduction in TNFalpha/z-VAD-FMK-induced necrosis after 24 hrs by Cell Titer-Glo luminescent cell ... | J Med Chem 60: 972-986 (2017) BindingDB Entry DOI: 10.7270/Q2QC05QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||