

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50229691 CHEMBL23118
SMILES: Cc1nnn(n1)C1CN2CCC1CC2
InChI Key: InChIKey=VPPCYMOGGLAYBU-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor (RAT) | BDBM50229691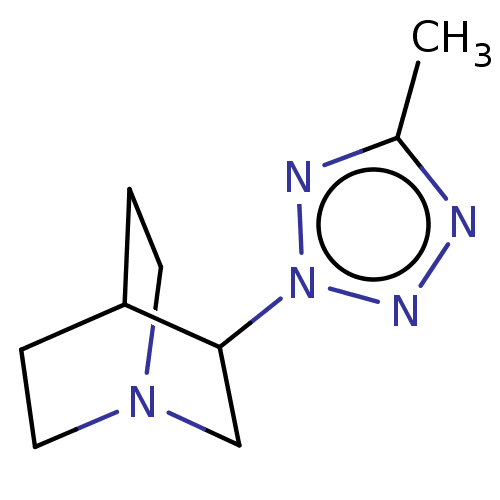 (CHEMBL23118) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested in vitro for its binding affinity to muscarinic acetylcholine receptor from rat cortical homogenates using [3H]QNB as radioligand | J Med Chem 35: 1280-90 (1992) BindingDB Entry DOI: 10.7270/Q2G1632Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor (RAT) | BDBM50229691 (CHEMBL23118) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description In vitro displacement of [3H]quinuclidinyl benzilate (QNB) from rat cerebral cortex muscarinic receptor. | J Med Chem 35: 2392-406 (1992) BindingDB Entry DOI: 10.7270/Q2TF00K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor (RAT) | BDBM50229691 (CHEMBL23118) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. | J Med Chem 35: 2392-406 (1992) BindingDB Entry DOI: 10.7270/Q2TF00K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor (RAT) | BDBM50229691 (CHEMBL23118) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested in vitro for its binding affinity to muscarinic acetylcholine receptor from rat cortical homogenates using [3H]OXO-M as radioliga... | J Med Chem 35: 1280-90 (1992) BindingDB Entry DOI: 10.7270/Q2G1632Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||