

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50229976 CHEMBL4098557
SMILES: CC(C)C[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CSCCC(=O)N2CN3CN(C2)C(=O)CCSC[C@H](NC(=O)[C@H](C)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CSCCC3=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O)NC(=O)[C@@H](N)CCCNC(N)=N
InChI Key: InChIKey=IAHJTJAHNSHYFK-IOBGLNQYSA-N
Data: 1 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor XII (Homo sapiens (Human)) | BDBM50229976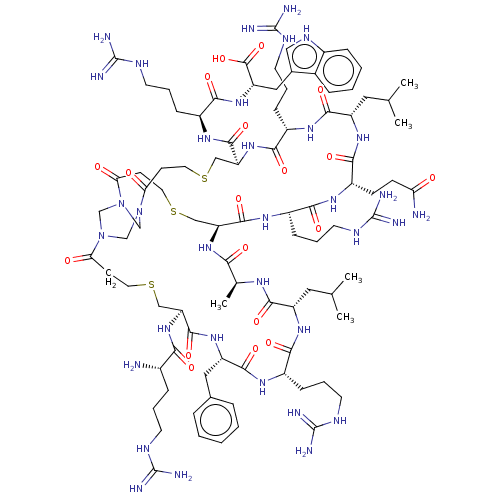 (CHEMBL4098557) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Polytechnique F�d�rale de Lausanne (EPFL) Curated by ChEMBL | Assay Description Inhibition of human beta factor 12a using fluorogenic substrate Boc-Gln-Gly-Arg-AMC preincubated for 10 mins followed by addition of substrate measur... | J Med Chem 60: 1151-1158 (2017) BindingDB Entry DOI: 10.7270/Q26D5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||