

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50230926 CHEMBL4083504
SMILES: COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2ncc(NC(=O)CN3CCOCC3)c(OC)c2c1
InChI Key: InChIKey=PESHAFCSMREEAN-UHFFFAOYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50230926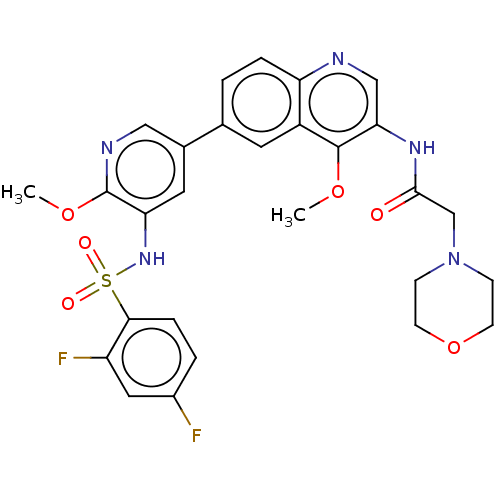 (CHEMBL4083504) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hangzhou Xixi Hospital Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal FLAG-tagged mTOR (1362-end residues) using ULight-4E-BP1 peptide substrate after 1 hr in presence of ATP b... | Eur J Med Chem 127: 509-520 (2017) BindingDB Entry DOI: 10.7270/Q2CZ39DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| p110α/p85α (Homo sapiens (Human)) | BDBM50230926 (CHEMBL4083504) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Hangzhou Xixi Hospital Curated by ChEMBL | Assay Description Inhibition of PI3K p110alpha/p85alpha (unknown origin) using PIP2 as substrate after 1 hr by luminescent kinase-Glo assay | Eur J Med Chem 127: 509-520 (2017) BindingDB Entry DOI: 10.7270/Q2CZ39DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50230926 (CHEMBL4083504) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Hangzhou Xixi Hospital Curated by ChEMBL | Assay Description Inhibition of PI3K p110delta (unknown origin) using PIP2 as substrate after 1 hr by ADP-Glo kinase assay | Eur J Med Chem 127: 509-520 (2017) BindingDB Entry DOI: 10.7270/Q2CZ39DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50230926 (CHEMBL4083504) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hangzhou Xixi Hospital Curated by ChEMBL | Assay Description Inhibition of recombinant full length His-tagged human PI3K p110gamma expressed in baculovirus expression system using PIP2 as substrate after 1 hr b... | Eur J Med Chem 127: 509-520 (2017) BindingDB Entry DOI: 10.7270/Q2CZ39DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| p110β/p85α (Homo sapiens (Human)) | BDBM50230926 (CHEMBL4083504) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Hangzhou Xixi Hospital Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal His6-tagged PI3K p110beta/p85alpha expressed in baculovirus infected Sf21 cells using PIP2 as ... | Eur J Med Chem 127: 509-520 (2017) BindingDB Entry DOI: 10.7270/Q2CZ39DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||