

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50233887 CHEMBL1511860
SMILES: C(Sc1nc[nH]n1)c1csc(Nc2ccccc2)n1
InChI Key: InChIKey=WLZCMHNSPJKOQN-UHFFFAOYSA-N
Data: 9 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50233887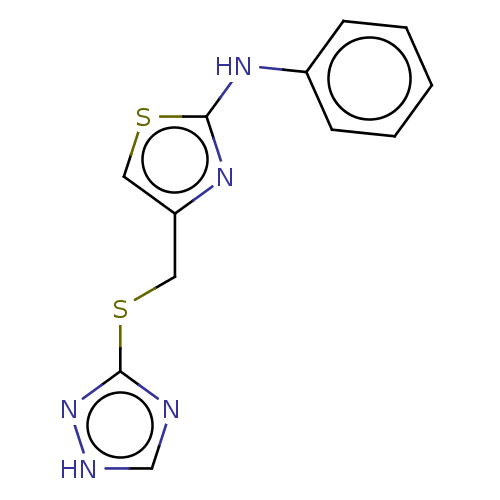 (CHEMBL1511860) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of APMA-activated recombinant human MMP-2 using Cy3-PLGLK(Cy5Q)AR-NH2 peptide as substrate measured after 40 mins by spectrofluorimetric m... | J Med Chem 60: 608-626 (2017) BindingDB Entry DOI: 10.7270/Q2W95CF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50233887 (CHEMBL1511860) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of APMA-activated recombinant human MMP-13 using Cy3-PLGLK(Cy5Q)AR-NH2 peptide as substrate measured after 40 mins by spectrofluorimetric ... | J Med Chem 60: 608-626 (2017) BindingDB Entry DOI: 10.7270/Q2W95CF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50233887 (CHEMBL1511860) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of APMA-activated recombinant human MMP-8 using Cy3-PLGLK(Cy5Q)AR-NH2 peptide as substrate measured after 40 mins by spectrofluorimetric m... | J Med Chem 60: 608-626 (2017) BindingDB Entry DOI: 10.7270/Q2W95CF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase 10 (Homo sapiens (Human)) | BDBM50233887 (CHEMBL1511860) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 8.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of APMA-activated recombinant human MMP-10 using Cy3-PLGLK(Cy5Q)AR-NH2 peptide as substrate measured after 40 mins by spectrofluorimetric ... | J Med Chem 60: 608-626 (2017) BindingDB Entry DOI: 10.7270/Q2W95CF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50233887 (CHEMBL1511860) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of APMA-activated recombinant human MMP-1 using Cy3-PLGLK(Cy5Q)AR-NH2 peptide as substrate measured after 40 mins by spectrofluorimetric m... | J Med Chem 60: 608-626 (2017) BindingDB Entry DOI: 10.7270/Q2W95CF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase 7 (Homo sapiens (Human)) | BDBM50233887 (CHEMBL1511860) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of APMA-activated recombinant human MMP-7 using Cy3-PLGLK(Cy5Q)AR-NH2 peptide as substrate measured after 40 mins by spectrofluorimetric m... | J Med Chem 60: 608-626 (2017) BindingDB Entry DOI: 10.7270/Q2W95CF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50233887 (CHEMBL1511860) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of APMA-activated recombinant human MMP-9 using Cy3-PLGLK(Cy5Q)AR-NH2 peptide as substrate measured after 40 mins by spectrofluorimetric m... | J Med Chem 60: 608-626 (2017) BindingDB Entry DOI: 10.7270/Q2W95CF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase 14 (Homo sapiens (Human)) | BDBM50233887 (CHEMBL1511860) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of APMA-activated recombinant human GST-tagged MMP-14 using Cy3-PLGLK(Cy5Q)AR-NH2 peptide as substrate measured after 40 mins by spectrofl... | J Med Chem 60: 608-626 (2017) BindingDB Entry DOI: 10.7270/Q2W95CF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50233887 (CHEMBL1511860) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of APMA-activated recombinant human MMP-3 using Cy3-PLGLK(Cy5Q)AR-NH2 peptide as substrate measured after 40 mins by spectrofluorimetric m... | J Med Chem 60: 608-626 (2017) BindingDB Entry DOI: 10.7270/Q2W95CF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||