

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50234040 CHEMBL4060089::US9765018, Example 114
SMILES: COc1ccc(cc1C(O)=O)-c1ccc(Oc2cccc3CCCCc23)c(NC(=O)Nc2ccccc2Cl)c1
InChI Key: InChIKey=OLUVZKHXNIKBIS-UHFFFAOYSA-N
Data: 10 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50234040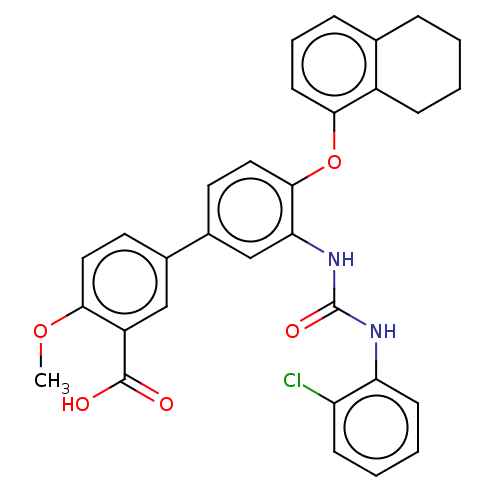 (CHEMBL4060089 | US9765018, Example 114) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | Bioorg Med Chem Lett 27: 582-585 (2017) BindingDB Entry DOI: 10.7270/Q2QN6914 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50234040 (CHEMBL4060089 | US9765018, Example 114) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) | Bioorg Med Chem Lett 27: 582-585 (2017) BindingDB Entry DOI: 10.7270/Q2QN6914 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50234040 (CHEMBL4060089 | US9765018, Example 114) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) | Bioorg Med Chem Lett 27: 582-585 (2017) BindingDB Entry DOI: 10.7270/Q2QN6914 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50234040 (CHEMBL4060089 | US9765018, Example 114) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using BZR as substrate | Bioorg Med Chem Lett 27: 582-585 (2017) BindingDB Entry DOI: 10.7270/Q2QN6914 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50234040 (CHEMBL4060089 | US9765018, Example 114) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company; Syngene International Limited; Bristol-Myers Squibb Company US Patent | Assay Description Human IDO1/HEK293 cells were seeded at 10,000 cells per 50 uL per well with RPMI/phenol red free media contains 10% FBS in a 384-well black wall clea... | US Patent US9765018 (2017) BindingDB Entry DOI: 10.7270/Q2KP849H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50234040 (CHEMBL4060089 | US9765018, Example 114) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells assessed as inhibition of kynurenine production preincubated with cells followed by IFN-g... | Bioorg Med Chem Lett 27: 582-585 (2017) BindingDB Entry DOI: 10.7270/Q2QN6914 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50234040 (CHEMBL4060089 | US9765018, Example 114) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2B6 (unknown origin) | Bioorg Med Chem Lett 27: 582-585 (2017) BindingDB Entry DOI: 10.7270/Q2QN6914 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50234040 (CHEMBL4060089 | US9765018, Example 114) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | Bioorg Med Chem Lett 27: 582-585 (2017) BindingDB Entry DOI: 10.7270/Q2QN6914 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50234040 (CHEMBL4060089 | US9765018, Example 114) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using BFC as substrate | Bioorg Med Chem Lett 27: 582-585 (2017) BindingDB Entry DOI: 10.7270/Q2QN6914 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50234040 (CHEMBL4060089 | US9765018, Example 114) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C8 (unknown origin) | Bioorg Med Chem Lett 27: 582-585 (2017) BindingDB Entry DOI: 10.7270/Q2QN6914 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||