

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50235372 CHEMBL3966307
SMILES: OC(=O)COC[C@H]1CC[C@@H](COC(=O)N(c2ccccc2)c2cccc(Cl)c2)CC1
InChI Key: InChIKey=DNBJSWUQGXMSOJ-HDICACEKSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTGIR (RAT) | BDBM50235372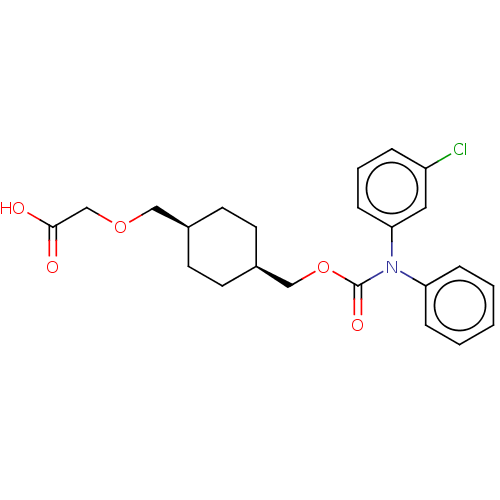 (CHEMBL3966307) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 272 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at recombinant rat IP receptor expressed in CHO-K1 cells assessed as increase in intracellular cAMP level after 1 hr incubation by H... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostanoid DP receptor (Homo sapiens (Human)) | BDBM50235372 (CHEMBL3966307) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.07E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at recombinant human DP1 receptor expressed in CHO-K1 cells assessed as increase in intracellular cAMP level after 1 hr incubation b... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostanoid IP receptor (Homo sapiens (Human)) | BDBM50235372 (CHEMBL3966307) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostanoid IP receptor (Homo sapiens (Human)) | BDBM50235372 (CHEMBL3966307) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at recombinant human IP receptor expressed in CHO-K1 cells assessed as increase in intracellular cAMP level after 1 hr incubation by... | J Med Chem 60: 913-927 (2017) Article DOI: 10.1021/acs.jmedchem.6b00871 BindingDB Entry DOI: 10.7270/Q2VX0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||