

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Nc1ncnn2c(ccc12)[C@@]1(O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O)C#N
InChI Key: InChIKey=DFVPCNAMNAPBCX-LTGWCKQJSA-N
PDB links: 1 PDB ID matches this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rous sarcoma virus integrase (Rous sarcoma virus (strain Prague C) (RSV-PrC)) | BDBM50236025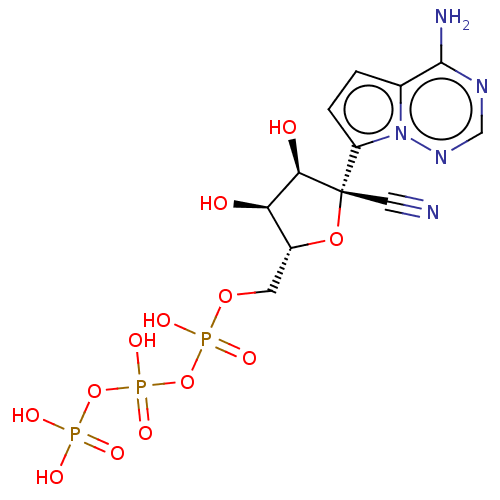 (CHEMBL2016761 | US11149049, No. C2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Standard RSV polymerase assays were conducted in the presence of 3 μL extract of RSV-infected cells in a reaction buffer containing 50 mM tris-a... | Citation and Details BindingDB Entry DOI: 10.7270/Q28P63PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 16) | BDBM50236025 (CHEMBL2016761 | US11149049, No. C2) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzyme activity of hepatitis C virus RNA polymerase (HCVpol) and human rhinovirus 16 RNA polymerase (HRV16pol) is measured as an incorporation of... | Citation and Details BindingDB Entry DOI: 10.7270/Q28P63PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM50236025 (CHEMBL2016761 | US11149049, No. C2) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzyme activity of hepatitis C virus RNA polymerase (HCVpol) and human rhinovirus 16 RNA polymerase (HRV16pol) is measured as an incorporation of... | Citation and Details BindingDB Entry DOI: 10.7270/Q28P63PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (DENV-1) | BDBM50236025 (CHEMBL2016761 | US11149049, No. C2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzyme activity of dengue virus NS5 polymerase domain (DENVpol, serotype 2, New Guinea C strain) was measured as an incorporation of tritiated NM... | Citation and Details BindingDB Entry DOI: 10.7270/Q28P63PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50236025 (CHEMBL2016761 | US11149049, No. C2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human DNA polymerase beta preincubated with enzyme followed by addition of dATP/dGTP/TTP as substrate in presence of [gamma... | J Med Chem 60: 1648-1661 (2017) Article DOI: 10.1021/acs.jmedchem.6b01594 BindingDB Entry DOI: 10.7270/Q2QZ2D7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase, mitochondrial (Homo sapiens (Human)) | BDBM50236025 (CHEMBL2016761 | US11149049, No. C2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human mitochondrial RNA polymerase after 30 mins in presence of [33P]GTP | J Med Chem 60: 1648-1661 (2017) Article DOI: 10.1021/acs.jmedchem.6b01594 BindingDB Entry DOI: 10.7270/Q2QZ2D7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50236025 (CHEMBL2016761 | US11149049, No. C2) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human DNA polymerase alpha preincubated with enzyme followed by addition of dATP/dGTP/TTP as substrate in presence of [gamm... | J Med Chem 60: 1648-1661 (2017) Article DOI: 10.1021/acs.jmedchem.6b01594 BindingDB Entry DOI: 10.7270/Q2QZ2D7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||