 Found 12 hits for monomerid = 50236531
Found 12 hits for monomerid = 50236531 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50236531
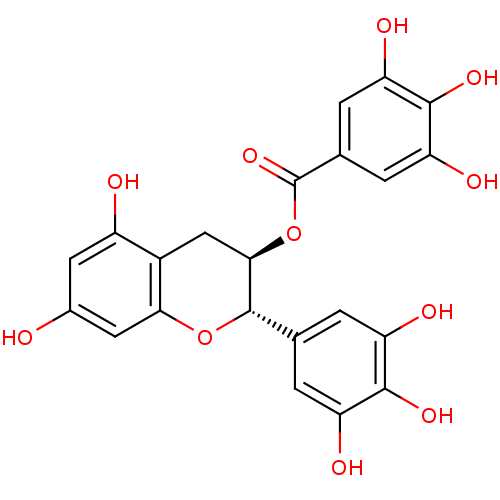
((-)-gallocatechin gallate | (2R,3S)-5,7-dihydroxy-...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 235 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of NLWAAQRYGRELRRMSD-K(FITC)-FVD from Bcl-2 (unknown origin) by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1007/s00044-009-9233-5
BindingDB Entry DOI: 10.7270/Q27M0BVB |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50236531

((-)-gallocatechin gallate | (2R,3S)-5,7-dihydroxy-...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Inhibition of MET kinase |
J Med Chem 52: 6543-5 (2009)
Article DOI: 10.1021/jm901330e
BindingDB Entry DOI: 10.7270/Q2M908RG |
More data for this
Ligand-Target Pair | |
Enoyl-acyl-carrier protein reductase
(Plasmodium falciparum) | BDBM50236531

((-)-gallocatechin gallate | (2R,3S)-5,7-dihydroxy-...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of FabI |
J Med Chem 49: 3345-53 (2006)
Article DOI: 10.1021/jm0600545
BindingDB Entry DOI: 10.7270/Q2639QJS |
More data for this
Ligand-Target Pair | |
3-hydroxyacyl-[acyl-carrier-protein] dehydratase
(Plasmodium falciparum) | BDBM50236531

((-)-gallocatechin gallate | (2R,3S)-5,7-dihydroxy-...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of FabZ |
J Med Chem 49: 3345-53 (2006)
Article DOI: 10.1021/jm0600545
BindingDB Entry DOI: 10.7270/Q2639QJS |
More data for this
Ligand-Target Pair | |
3-oxoacyl-acyl-carrier protein reductase
(Plasmodium falciparum) | BDBM50236531

((-)-gallocatechin gallate | (2R,3S)-5,7-dihydroxy-...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of FabG |
J Med Chem 49: 3345-53 (2006)
Article DOI: 10.1021/jm0600545
BindingDB Entry DOI: 10.7270/Q2639QJS |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50236531

((-)-gallocatechin gallate | (2R,3S)-5,7-dihydroxy-...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128675
BindingDB Entry DOI: 10.7270/Q2639TS5 |
More data for this
Ligand-Target Pair | |
Glucose-6-phosphate 1-dehydrogenase
(Saccharomyces cerevisiae S288c) | BDBM50236531

((-)-gallocatechin gallate | (2R,3S)-5,7-dihydroxy-...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation
Curated by ChEMBL
| Assay Description
Inhibition of yeast G6PD |
Bioorg Med Chem 16: 3580-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.030
BindingDB Entry DOI: 10.7270/Q2F190K1 |
More data for this
Ligand-Target Pair | |
Nonstructural protein 3
(Zika virus) | BDBM50236531

((-)-gallocatechin gallate | (2R,3S)-5,7-dihydroxy-...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21+/m1/s1 | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of Zika virus NS2B (1421 to 1469 residues) - NS3 (1503 to 1688 residues) expressed in Escherichia coli BL21(DE3) cells using Dabcyl-KTSAVL... |
J Med Chem 63: 470-489 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00775
BindingDB Entry DOI: 10.7270/Q2RX9GFS |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50236531

((-)-gallocatechin gallate | (2R,3S)-5,7-dihydroxy-...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 187 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Agricultural and Food Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of purified human 20S proteasome assessed as decrease in AMC hydrolysis using Suc-Leu-Leu-Val-Tyr-AMC as sub... |
Eur J Med Chem 167: 291-311 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.044
BindingDB Entry DOI: 10.7270/Q2ST7TB3 |
More data for this
Ligand-Target Pair | |
Alpha-synuclein
(Homo sapiens (Human)) | BDBM50236531

((-)-gallocatechin gallate | (2R,3S)-5,7-dihydroxy-...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human alpha-synuclein filament formation expressed in Escherichia coli BL21(DE3) cells incubated for 72 hrs by thioflavin S based fluor... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2019.01.045
BindingDB Entry DOI: 10.7270/Q2H998V9 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50236531

((-)-gallocatechin gallate | (2R,3S)-5,7-dihydroxy-...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Montclair State University
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 (unknown origin) by luciferase refolding assay |
Bioorg Med Chem Lett 24: 2263-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.088
BindingDB Entry DOI: 10.7270/Q2RF5WK6 |
More data for this
Ligand-Target Pair | |
6-phosphogluconate dehydrogenase, decarboxylating
(Homo sapiens (Human)) | BDBM50236531

((-)-gallocatechin gallate | (2R,3S)-5,7-dihydroxy-...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 6PGD |
Bioorg Med Chem 16: 3580-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.030
BindingDB Entry DOI: 10.7270/Q2F190K1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data