

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50236545 CHEMBL4087125
SMILES: Oc1ccc(cc1)-c1c[nH]c2ncc(cc12)-c1ccc(cc1)-c1nnn[nH]1
InChI Key: InChIKey=XVVPNHZWUXCHJE-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dual-specificity tyrosine-phosphorylation regulated kinase 1A (Homo sapiens (Human)) | BDBM50236545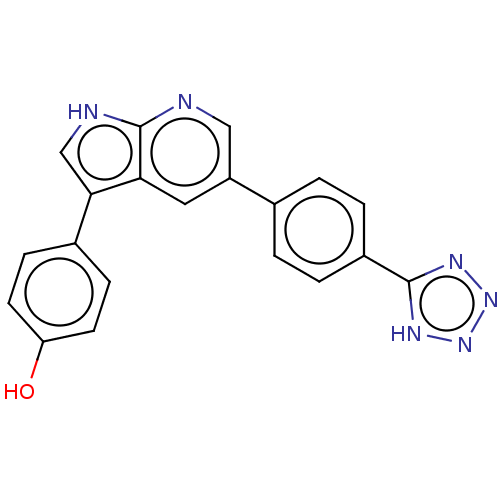 (CHEMBL4087125) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
QIMR Berghofer Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of DYRK1A (unknown origin) using woodtide as substrate after 10 mins in presence of [gamma-33P]ATP by microbeta scintillation counting | J Med Chem 60: 2052-2070 (2017) Article DOI: 10.1021/acs.jmedchem.6b01840 BindingDB Entry DOI: 10.7270/Q2ST7S3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM50236545 (CHEMBL4087125) | UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
QIMR Berghofer Medical Research Institute Curated by ChEMBL | Assay Description Antagonistic activity against human androgen receptor (hAR) in CV-1 cells using cotransfection assay | J Med Chem 60: 2052-2070 (2017) Article DOI: 10.1021/acs.jmedchem.6b01840 BindingDB Entry DOI: 10.7270/Q2ST7S3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1/CLK4 (Homo sapiens (Human)) | BDBM50236545 (CHEMBL4087125) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a |
QIMR Berghofer Medical Research Institute Curated by ChEMBL | Assay Description Antagonistic activity against human androgen receptor (hAR) in CV-1 cells using cotransfection assay | J Med Chem 60: 2052-2070 (2017) Article DOI: 10.1021/acs.jmedchem.6b01840 BindingDB Entry DOI: 10.7270/Q2ST7S3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual-specificity tyrosine-phosphorylation regulated kinase 2 (Homo sapiens (Human)) | BDBM50236545 (CHEMBL4087125) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 408 | n/a | n/a | n/a | n/a | n/a | n/a |
QIMR Berghofer Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of DYRK2 (unknown origin) using woodtide as substrate after 40 mins in presence of [gamma-33P]ATP by microbeta scintillation counting | J Med Chem 60: 2052-2070 (2017) Article DOI: 10.1021/acs.jmedchem.6b01840 BindingDB Entry DOI: 10.7270/Q2ST7S3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||