

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50237158 CHEMBL4071868
SMILES: CCCN(CCCCOc1ccc2c(\C=N\O)cnn2c1)[C@H]1CCc2c(O)cccc2C1
InChI Key: InChIKey=HBUKKEFQANLSNF-XLKFHUSGSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50237158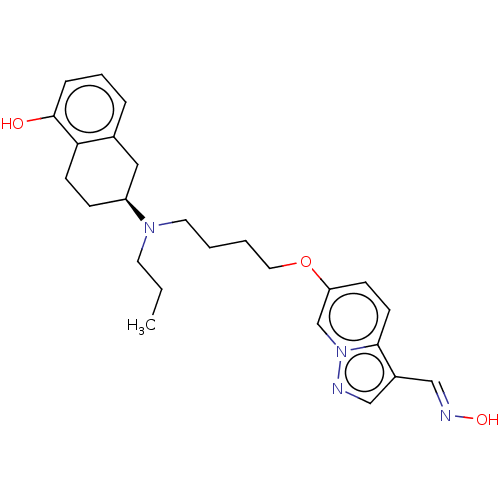 (CHEMBL4071868) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at human Dopamine D3 receptor expressed in HEK293T cell membranes coexpressing GalphaoA incubated for 30 mins measured after 75 mins... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237158 (CHEMBL4071868) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D2S receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237158 (CHEMBL4071868) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Inhibition of FPP incorporation into biotinylated K-Ras-derived peptide by human farnesyl transferase | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50237158 (CHEMBL4071868) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human Dopamine D4 receptor expressed in CHO cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50237158 (CHEMBL4071868) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human Dopamine D1 receptor expressed in HEK293T cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50237158 (CHEMBL4071868) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5-HT2A receptor expressed in HEK293T cell membranes after 2 hrs by scintillation counting analysis | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237158 (CHEMBL4071868) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at N-terminal flag-tagged D2S receptor (unknown origin) expressed in HEK293 cells assessed as induction of renilla luciferase 2-tagg... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237158 (CHEMBL4071868) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at N-terminal flag-tagged D2S receptor (unknown origin) expressed in HEK293 cells coexpressing renilla luciferase 2-tagged Galphai1 ... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237158 (CHEMBL4071868) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at N-terminal flag-tagged D2S receptor (unknown origin) expressed in HEK293 cells coexpressing renilla luciferase 2-tagged Galphai3 ... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237158 (CHEMBL4071868) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at N-terminal flag-tagged D2S receptor (unknown origin) expressed in HEK293 cells coexpressing renilla luciferase 2-tagged GalphaoB ... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237158 (CHEMBL4071868) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Concentration required for the inhibitory activity against human Farnesyltransferase | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237158 (CHEMBL4071868) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Concentration required for the inhibitory activity against human Farnesyltransferase | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237158 (CHEMBL4071868) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at N-terminal flag-tagged D2S receptor (unknown origin) expressed in HEK293 cells assessed as induction of renilla luciferase 2-tagg... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237158 (CHEMBL4071868) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at ARMS2-PK2 tagged D2S receptor (unknown origin) expressed in HEK293 cells assessed as induction of EA-tagged beta-arrestin-2 recru... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||